 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,2,4-Trimethylpentane[1] | |
| Identifiers | |
3D model (JSmol) |
|
| 1696876 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.964 |
| EC Number |
|
| MeSH | 2,2,4-trimethylpentane |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1262 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H18 | |
| Molar mass | 114.232 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | petroleum-like |
| Density | 0.692 g cm−3 |
| Melting point | −107.38 °C; −161.28 °F; 165.77 K |
| Boiling point | 99.30 °C; 210.74 °F; 372.45 K |
| log P | 4.373 |
| Vapor pressure | 5.5 kPa (at 21 °C) |
Henry's law constant (kH) |
3.0 nmol Pa−1 kg−1 |
| UV-vis (λmax) | 210 nm |
| -98.34·10−6 cm3/mol | |
Refractive index (nD) |
1.391 |
| Thermochemistry | |
Heat capacity (C) |
242.49 J K−1 mol−1 |
Std molar entropy (S⦵298) |
328.03 J K−1 mol−1 |
Std enthalpy of formation (ΔfH⦵298) |
−260.6 to −258.0 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) |
−5462.6 to −5460.0 kJ mol−1 |
| Hazards | |
| GHS labelling: | |
  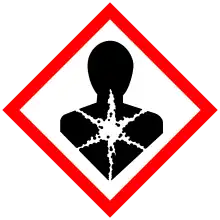 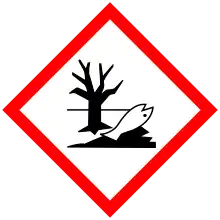 | |
| Danger | |
| H225, H304, H315, H336, H410 | |
| P210, P261, P273, P301+P310, P331 | |
| NFPA 704 (fire diamond) | |
| Flash point | −12 °C (10 °F; 261 K) |
| 396 °C (745 °F; 669 K) | |
| Explosive limits | 1.1–6.0% |
| Related compounds | |
Related alkanes |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
2,2,4-Trimethylpentane, also known as isooctane or iso-octane, is an organic compound with the formula (CH3)3CCH2CH(CH3)2. It is one of several isomers of octane (C8H18). This particular isomer is the standard 100 point on the octane rating scale (the zero point is n-heptane). It is an important component of gasoline, frequently used in relatively large proportions (around 10%) to increase the knock resistance of fuel.[2][3]
Strictly speaking, if the standard meaning of ‘iso’ is followed, the name isooctane should be reserved for the isomer 2-methylheptane. However, 2,2,4-trimethylpentane is by far the most important isomer of octane and historically it has been assigned this name.[4]
Production
Isooctane is produced on a massive scale in the petroleum industry by alkylation of isobutene with isobutane. This process is conducted in alkylation units in the presence of acid catalysts.[5]
It can also be produced from isobutylene by dimerization using an Amberlyst catalyst to produce a mixture of iso-octenes. Hydrogenation of this mixture produces 2,2,4-trimethylpentane.[6]
History
Engine knocking is an unwanted process that can occur during high compression ratios in internal combustion engines. In 1926 Graham Edgar added different amounts of n-heptane and 2,2,4-trimethylpentane to gasoline, and discovered that the knocking stopped when 2,2,4-trimethylpentane was added. This work was the origin of the octane rating scale.[7] Test motors using 2,2,4-trimethylpentane gave a certain performance that was standardized as 100 octane. The same test motors, run in the same fashion, using heptane, gave a performance which was standardized as 0 octane. All other compounds and blends of compounds then were graded against these two standards and assigned octane numbers.
Safety
In common with all hydrocarbons, 2,2,4-trimethylpentane is flammable.[8]
See also
References
- ↑ "2,2,4-trimethylpentane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 11 March 2012.
- ↑ Werner Dabelstein; Arno Reglitzky; Andrea Schütze; Klaus Reders (2007). "Automotive Fuels". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a16_719.pub2. ISBN 978-3527306732.
- ↑ Richardson, KA; Wilmer, JL; Smith-Simpson, D; Skopek, TR (February 1986). "Assessment of the genotoxic potential of unleaded gasoline and 2,2,4-trimethylpentane in human lymphoblasts in vitro". Toxicology and Applied Pharmacology. 82 (2): 316–22. doi:10.1016/0041-008x(86)90207-3. PMID 3945956.
- ↑ Clayden, Jonathan (2005). Organic chemistry (Reprinted (with corrections). ed.). Oxford [u.a.]: Oxford Univ. Press. pp. 315. ISBN 978-0-19-850346-0.
- ↑ Bipin V. Vora; Joseph A. Kocal; Paul T. Barger; Robert J. Schmidt; James A. Johnson (2003). "Alkylation". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.0112112508011313.a01.pub2. ISBN 0471238961.
- ↑ Dimerization of isobutylene, Amberlyst.com
- ↑ Fuels and lubricants handbook, Volume 1, George E. Totten, Steven R. Westbrook, Rajesh J. Shah, page 62
- ↑ 2,2,4-Trimethylpentane, Integrated Risk Information System, United States Environmental Protection Agency

