 | |
 | |
| Names | |
|---|---|
| IUPAC name
3,3′-Diiodo-DL-thyronine | |
| Systematic IUPAC name
2-Amino-3-[4-(4-Hydroxy-3-iodophenoxy)-3-iodophenyl]propanoic acid | |
| Other names
O-(4-Hydroxy-3-iodophenyl)-3-iodotyrosine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| MeSH | 3,3'-diiodothyronine |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C15H13I2NO4 | |
| Molar mass | 525.077 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
3,3'-Diiodothyronine, also known as 3,3'-T2, is a metabolite of thyroid hormone.
It is formed from the breakdown of triiodothyronine. Levels can be affected in certain disease states.[1]
Reactions
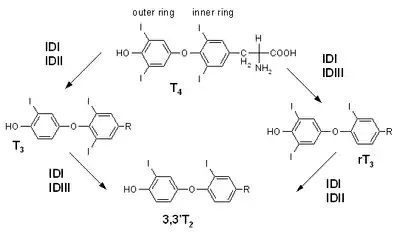
Synthesis of T2 from T3, and from reverse T3
References
- ↑ Pinna G; Hiedra L; Meinhold H; et al. (September 1998). "3,3'-Diiodothyronine concentrations in the sera of patients with nonthyroidal illnesses and brain tumors and of healthy subjects during acute stress". J. Clin. Endocrinol. Metab. 83 (9): 3071–7. doi:10.1210/jcem.83.9.5080. PMID 9745405.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.