 | |
| Names | |
|---|---|
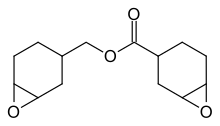
| Other names
7-Oxabicyclo[4.1.0]hept-3-ylmethyl-7-oxabicyclo[4.1.0]heptane-3-carboxylate | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | ECC |
| ChemSpider | |
| ECHA InfoCard | 100.017.463 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H20O4 | |
| Molar mass | 252.310 g·mol−1 |
| Appearance | Colorless liquid[1] |
| Density | 1.17 g·cm−3[1] |
| Melting point | −37 °C (−35 °F; 236 K)[1] |
| Slightly soluble (13.85 g·l−1 (20 °C))[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
3,4-Epoxycyclohexylmethyl-3',4'-epoxycyclohexane carboxylate (ECC) is a cycloaliphatic epoxy resin which is used in many industrial applications. It reacts by cationic polymerization using thermolatent photoinitiators to form crosslinked insoluble thermosets. Formulations based on cycloaliphatic epoxy resins such as ECC are known to form by curing thermosets with high heat and chemical resistance and good adhesion.[2]
History
The homopolymerization of ECC is based on radiation curing, which proceeds via a photochemical formation of a super acid and subsequent cationic polymerization. This was the first time realized in the 1970s.[3]
Fabrication
ECC can be prepared via Tishchenko reaction of tetrahydrobenzaldehyde and subsequent epoxidation with a peracid.[4]
Properties
ECC has a dynamic viscosity of 400 mPa·s at 25 °C.[2]
Reactivity
For homopolymerization of ECC 1.5 to 3 wt. % of an initiator are added. Above 3 wt% initiator no further acceleration was found, increasing proportions of initiators, however, increase the brittleness of the formed thermoset. After a photopolymerization usually still a thermal post-curing is necessary for a complete reaction.[5]
It is known that the reactivity of the monomer is lower than it could be, since the contained ester group can react with the reactive, polymerizing chain end and stabilize it. It therefore reacts significantly slower than other molecules without ester group.[2][6] ECC polymerizes also much slower than radical monomers. It is therefore the object of research to find cationic polymerizable monomers with higher polymerization rate but same performance.[2]
Crosslinking
Cationically crosslinked ECC is used in a variety of industrial applications, due to its low viscosity, excellent electrical properties and high reliability among others as an electrical insulator, as coating and adhesive or as printing ink.[7] Homopolymerized ECC, however, is extremely brittle, which is disadvantageous. This problem can be addressed by integration of elastomer particles in the epoxy matrix, such as rubber or silicone, by integration of inorganic fillers[8] or by plasticization due to polymerization in the presence of polyester polyols.[9] The latter are covalently integrated via the monomer-activated mechanism into the polymer network.[10]
References
- 1 2 3 4 Record of 3,4-Epoxycyclohexylmethyl-3',4'-epoxycyclohexancarboxylat in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 1 January 2015.
- 1 2 3 4 Sasaki, Hiroshi (February 2007). "Curing properties of cycloaliphatic epoxy derivatives". Progress in Organic Coatings. 58 (2–3): 227–230. doi:10.1016/j.porgcoat.2006.09.030.
- ↑ Crivello, J. V.; Lam, J. H. W. (October 1978). "Dye-sensitized photoinitiated cationic polymerization". Journal of Polymer Science: Polymer Chemistry Edition. 16 (10): 2441–2451. doi:10.1002/pol.1978.170161004.
- ↑ Dillman, Brian; Jessop, Julie L. P. (2013-05-01). "Chain transfer agents in cationic photopolymerization of a bis-cycloaliphatic epoxide monomer: Kinetic and physical property effects". Journal of Polymer Science Part A: Polymer Chemistry. 51 (9): 2058–2067. doi:10.1002/pola.26595.
- ↑ Atsushi Udagawa; Yasuhiko Yamamoto; Yoshio Inoue; Riichirô Chûjô (January 1991). "Dynamic mechanical properties of cycloaliphatic epoxy resins cured by ultra-violet- and heat-initiated cationic polymerizations". Polymer. 32 (15): 2779–2784. doi:10.1016/0032-3861(91)90108-U.
- ↑ Crivello, James V.; Varlemann, Ulrike (October 1995). "Mechanistic study of the reactivity of 3,4-epoxycyclohexylmethyl 3′,4′-epoxycyclohexancarboxylate in photoinitiated cationic polymerizations". Journal of Polymer Science Part A: Polymer Chemistry. 33 (14): 2473–2486. doi:10.1002/pola.1995.080331421.
- ↑ Cristina Mas; Ana Mantecón; Angels Serra; Xavier Ramis & Josep Maria Salla (2005-06-01). "Improved thermosets obtained from cycloaliphatic epoxy resins and γ-butyrolactone with lanthanide triflates as initiators. I. Study of curing by differential scanning calorimetry and Fourier transform infrared". Journal of Polymer Science Part A: Polymer Chemistry. 43 (11): 2337–2347. doi:10.1002/pola.20711.
- ↑ Lützen, Hendrik; Bitomsky, Peter; Rezwan, Kurosch; Hartwig, Andreas (January 2013). "Partially crystalline polyols lead to morphology changes and improved mechanical properties of cationically polymerized epoxy resins". European Polymer Journal. 49 (1): 167–176. doi:10.1016/j.eurpolymj.2012.10.015.
- ↑ Spyrou, Emmanouil (November 2001). "Radiation initiated cationic polymerization with tailor-made polyesters". Progress in Organic Coatings. 43 (1–3): 25–31. doi:10.1016/S0300-9440(01)00240-5.
- ↑ Yagci, Yusuf; Schnabel, Wolfram (1999-09-01). "On the mechanism of photoinitiated cationic polymerization in the presence of polyols". Die Angewandte Makromolekulare Chemie. 270 (1): 38–41. doi:10.1002/(SICI)1522-9505(19990901)270:1<38::AID-APMC38>3.0.CO;2-S.
Literature
- Ellis, Bryan, ed. (2007). Polymers : a property database (2nd ed.). Boca Raton, Fla.: CRC. p. 150. ISBN 978-0-8493-3940-0.