Iodotoluenes are aryl iodides based on toluene in which at least one aromatic hydrogen atom is replaced with an iodine atom. They have the general formula C7H8–nIn, where n = 1–5 is the number of iodine atoms.
Monoiodotoluene
Monoiodotoluenes are iodotoluenes containing one iodine atom. There are three isomers, each with the formula C7H7I.
Properties
The isomers differ in the location of the iodine, but have the same chemical formula.
| Monoiodotoluene isomers[1][2][3] | ||||
|---|---|---|---|---|
| General | ||||
| Common name | o-iodotoluene | m-iodotoluene | p-iodotoluene | |
| Structure | 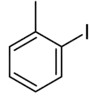 |
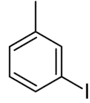 |
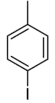 | |
| Systematic name | 1-iodo-2-methylbenzene | 1-iodo-3-methylbenzene | 1-iodo-4-methylbenzene | |
| Molecular formula | C7H7I (C6H4ICH3) | |||
| Molar mass | 218.03 g/mol | |||
| Appearance | Clear dark brown liquid | white to yellow solid | ||
| CAS number | [615-37-2] | [625-95-6] | [624-31-7] | |
| Properties | ||||
| Melting point | 35 °C (95 °F; 308 K)[4] | |||
| Boiling point | 211-212 °C (412-414 °F; 484-485 K)[4] | 211.5 °C (412.7 °F; 484.7 K)[5] | ||
Benzyl iodide is an isomer, which has a iodine substituted for one of the hydrogens of toluene's methyl group, and it is sometimes named α-bromoiodine.
Preparation
A laboratory route to o- and p-iodotoluene proceeds from toluene, which is treated with a mixture of iodine and nitric acid in an electrophilic aromatic substitution. The resulting mixture of o and p-iodotoluene is then separated by fractional freezing; cooling the mixture in an ice bath results in solidification of p-iodotoluene, which can then be isolated by filtration, while the o-iodotoluene remains behind as a liquid.[4]
Uses
Iodotoluenes are precursors to many organic building blocks. For example, the methyl group of o-iodotoluene and p-iodotoluene may be oxidized using potassium permanganate to form 2-iodobenzoic acid and 4-iodobenzoic acid, respectively.[6]
See also
References
- ↑ "2-Iodotoluene". PubChem. National Center for Biotechnology Information. Retrieved January 19, 2023.
- ↑ "3-Iodotoluene". PubChem. National Center for Biotechnology Information. Retrieved January 19, 2023.
- ↑ "4-Iodotoluene". PubChem. National Center for Biotechnology Information. Retrieved January 19, 2023.
- 1 2 3 Datta, Rasik Lal; Chatterjee, Nihar Ranjan (1917). "Halogenation. Xv. Direct Iodination of Hydrocarbons by Means of Iodine and Nitric Acid". Journal of the American Chemical Society. 39 (3): 435–441. doi:10.1021/ja02248a012.
- ↑ "4-Iodotoluene". Sigma Aldrich. Retrieved January 31, 2023.
- ↑ Varma, P. S.; Panickerp, P. B. (1928). "Influence of substitution on the oxidation of side chains in the benzene nucleus". Proc. 15th Indian Sci. Cong.