 | |
| Names | |
|---|---|
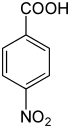
| Preferred IUPAC name
4-Nitrobenzoic acid | |
| Other names
p-Nitrobenzoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.479 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H5NO4 | |
| Molar mass | 167.1189 g/mol[1] |
| Appearance | Light yellow crystalline powder[2] |
| Density | 1.58[2] |
| Melting point | 237 °C (459 °F; 510 K)[2] |
| Boiling point | Sublimes[2] |
| <0.1 g/100 mL at 26 °C [3] | |
| Acidity (pKa) | 3.41 (in water),[4] 9.1 (in DMSO)[5] |
| -78.81·10−6 cm3/mol | |
| Related compounds | |
Related compounds |
Benzoic acid Nitrobenzene Anthranilic acid 3,5-Dinitrobenzoic acid, 3-Nitrobenzoic acid, 2-Nitrobenzoic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
4-Nitrobenzoic acid is an organic compound with the formula C6H4(NO2)CO2H. It is a pale yellow solid. It is a precursor to 4-nitrobenzoyl chloride, the precursor to the anesthetic procaine and folic acid. It is also a precursor to 4-aminobenzoic acid.[6]
Production
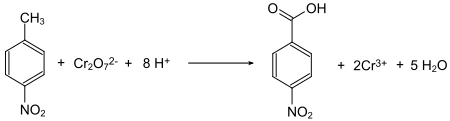
4-Nitrobenzoic acid is prepared by oxidation of 4-nitrotoluene using oxygen or dichromate as oxidants.[7]
Alternatively, it has been prepared by nitration of polystyrene followed by oxidation of the alkyl substituent. This method proceeds with improved para/ortho selectivity owing to the steric protection of the ortho positions by the polymer backbone.
Safety
References
- ↑ "4-nitrobenzoic acid - PubChem Public Chemical Database". Retrieved 11 April 2010.
- 1 2 3 4 "Safety data for p-nitrobenzoic acid". Archived from the original on 27 May 2008. Retrieved 11 April 2010.
- ↑ "p-Nitrobenzoic acid". Archived from the original on 7 May 2010. Retrieved 11 April 2010.
- ↑ "Dissociation Constants Of Organic Acids And Bases". Retrieved 11 April 2010.
- ↑ "Bordwell pKa Table (Acidity in DMSO)". Retrieved 11 April 2010.
- ↑ Takao Maki; Kazuo Takeda (2002). "Benzoic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_555. ISBN 3527306730..
- ↑ O. Kamm; A. O. Matthews (1922). "p-Nitrobenzoic Acid". Org. Synth. 2: 53. doi:10.15227/orgsyn.002.0053.
- ↑ "Material Safety Data Sheet - P-nitrobenzoic acid MSDS". Archived from the original on 7 August 2011. Retrieved 11 April 2010.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.