 | |
| Names | |
|---|---|
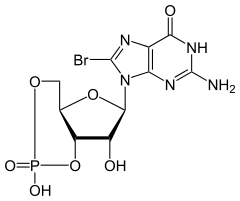
| IUPAC name
8-Bromoguanosine 3′,5′-(hydrogen phosphate) | |
| Preferred IUPAC name
(4aR,6R,7R,7aS)-6-(2-Amino-8-bromo-6-oxo-1,6-dihydro-9H-purin-9-yl)-2,7-dihydroxytetrahydro-2H,4H-2λ5-furo[3,2-d][1,3,2]dioxaphosphinin-2-one | |
| Other names
8-Bromocyclic GMP;8-Bromo-cGMP; 8-Br-cyclic GMP; 8-Br-cGMP | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H11BrN5O7P | |
| Molar mass | 424.104 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
8-Bromoguanosine 3′,5′-cyclic monophosphate is a brominated derivative of cyclic guanosine monophosphate (cGMP).[1] It acts as an activator of cGMP-dependent protein kinases.[2]
See also
- 8-Bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP)
References
- ↑ Rapoport, RM; Draznin, MB; Murad, F (Nov 1982). "Sodium nitroprusside-induced protein phosphorylation in intact rat aorta is mimicked by 8-bromo cyclic GMP" (Free full text). Proceedings of the National Academy of Sciences of the United States of America. 79 (21): 6470–4. Bibcode:1982PNAS...79.6470R. doi:10.1073/pnas.79.21.6470. ISSN 0027-8424. PMC 347148. PMID 6292902.
- ↑ 8-Bromoguanosine-3′,5′-cyclic monophosphate ( 8-Br-cGMP )
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.