 | |
| Clinical data | |
|---|---|
| Other names | AT-527, AT-511 |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
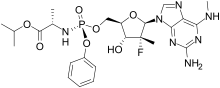
| Formula | C24H33FN7O7P |
| Molar mass | 581.542 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Bemnifosbuvir (AT-527, RO7496998) is an antiviral drug invented by Atea Pharmaceuticals and licensed to Roche for clinical development, a novel nucleotide analog prodrug originally developed for the treatment of hepatitis C.[1][2] Bemnifosbuvir is the orally bioavailable hemisulfate salt of AT-511, which is metabolized in several steps to the active nucleotide triphosphate AT-9010, acting as an RNA polymerase inhibitor and thereby interfering with viral replication. Bemnifosbuvir has been researched for the treatment of coronavirus diseases such as that produced by SARS-CoV-2.[3] It showed good results in early clinical trials but had inconsistent results at later stages, so the planned Phase 3 trials are being redesigned and results are not expected until late 2022.[4][5]
See also
References
- ↑ Berliba E, Bogus M, Vanhoutte F, Berghmans PJ, Good SS, Moussa A, et al. (September 2019). "Safety, pharmacokinetics and antiviral activity of AT-527, a novel purine nucleotide prodrug, in HCV-infected subjects with and without cirrhosis". Antimicrobial Agents and Chemotherapy. 63 (12). doi:10.1128/AAC.01201-19. PMC 6879261. PMID 31570394.
- ↑ Good SS, Moussa A, Zhou XJ, Pietropaolo K, Sommadossi JP (2020). "Preclinical evaluation of AT-527, a novel guanosine nucleotide prodrug with potent, pan-genotypic activity against hepatitis C virus". PLOS ONE. 15 (1): e0227104. Bibcode:2020PLoSO..1527104G. doi:10.1371/journal.pone.0227104. PMC 6949113. PMID 31914458.
- ↑ Good SS, Westover J, Jung KH, Zhou XJ, Moussa A, La Colla P, et al. (March 2021). "AT-527, a Double Prodrug of a Guanosine Nucleotide Analog, Is a Potent Inhibitor of SARS-CoV-2 In Vitro and a Promising Oral Antiviral for Treatment of COVID-19". Antimicrobial Agents and Chemotherapy. 65 (4). doi:10.1128/AAC.02479-20. PMC 8097421. PMID 33558299.
- ↑ Lowe D (19 October 2021). "AT-527 Fails a Phase II". In the Pipeline. Science.org.
- ↑ Fidler B, Gardner J (19 October 2021). "Atea, Roche change plans for oral COVID-19 drug after trial setback". Biopharmadive.com.