 | |
 | |
| Names | |
|---|---|
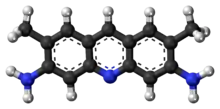
| Preferred IUPAC name
2,7-Dimethylacridine-3,6-diamine | |
| Other names
2,7-Dimethylproflavine Acridine yellow G | |
| Identifiers | |
3D model (JSmol) |
|
| 5-22-11-00340 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.947 |
| EC Number |
|
| MeSH | Acridine+yellow |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H15N3 | |
| Molar mass | 273.30 g/mol |
| Appearance | Brown/red crystals |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H302, H312, H315, H319, H332, H335, H351 | |
| P201, P202, P261, P264, P270, P271, P280, P281, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P308+P313, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Acridine yellow, also known as acridine yellow G, acridine yellow H107, basic yellow K, and 3,6-diamino-2,7-dimethylacridine, is a yellow dye with strong bluish-green fluorescence. It is a derivate of acridine. In histology, it is used as a fluorescent stain, and as a fluorescent probe for non-invasive measurements of cytoplasmic pH changes in whole cells. It is also used as a topical antiseptic. It is usually available as a hydrochloride salt. Acridine yellow damages DNA and is used as a mutagen in microbiology.
Acridine yellow is similar to acridine orange.
According to a publication by Karl Drechsler, a student of Guido Goldschmiedt at the Imperial and Royal University of Vienna, Moriz Freund discovered the substance in 1896 during experiments at the University of Prague. Drechsler was then able to produce the substance in larger quantities and subsequently also examine it more closely.[1]
References
- ↑ "ÖNB-ANNO - Monatshefte für Chemie". anno.onb.ac.at. Retrieved 2022-10-02.
External links
