 | |
 | |
| Names | |
|---|---|
| IUPAC name
Diammonium thiosulfate | |
| Other names
Ammonium thiosulphate, ATS | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.029.074 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
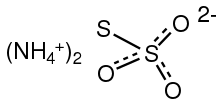
| [NH4]2S2O3 | |
| Molar mass | 148.20 g·mol−1 |
| Appearance | colorless or white, hygroscopic solid |
| Density | 1.679 g/cm3 |
| Melting point | decomposes at 100 °C |
| 173 g/100 mL (20 °C) | |
| Solubility | slightly soluble in acetone insoluble in alcohol |
| Structure | |
| monoclinic | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
2980 mg/kg (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Ammonium thiosulfate (ammonium thiosulphate in British English) is an inorganic compound with the formula [NH4]2S2O3. It is white crystalline solid with ammonia odor, readily soluble in water, slightly soluble in acetone and insoluble in ethanol and diethyl ether.[1]
Production
It is produced by treating ammonium sulfite with sulfur at temperatures between 85 and 110 °C:[2]
- [NH4]2SO3 + S → [NH4]2S2O3
Applications
Ammonium thiosulfate is used in photographic fixer. It is a so-called rapid fixer, acting more quickly than sodium thiosulfate fixers.[3] Fixation involves these chemical reactions (illustrated for silver bromide):[4]
- AgBr + 2 [NH4]2S2O3 → [NH4]3[Ag(S2O3)2] + [NH4]Br
- AgBr + 3 [NH4]2S2O3 → [NH4]5[Ag(S2O3)3] + [NH4]Br
Ammonium thiosulfate is also used for leaching of gold and silver. It works with presence of copper as a catalyst here. This process is a nontoxic alternative gold cyanidation.[5] The advantage to ammonium thiosulfate is that the pyrolysis of its silver complexes leaves a residue solely of silver sulfide, in contrast to complexes derived from sodium thiosulfate.[2]
Other
Ammonium thiosulfate can be used as a fertilizer.[6] As suggested by some research studies, it can also be used as an additive to coal-waste mixtures to reduce formation of dioxins and furans during combustion.[7]
Safety
See also
References
- ↑ MSDS - Ammonium Thiosulfate
- 1 2 3 J. J. Barbera; A. Metzger; M. Wolf (2012). "Sulfites, Thiosulfates, and Dithionites". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_477. ISBN 978-3527306732.
- ↑ "Praní černobílých filmů a papírů". Archived from the original on 2012-03-27. Retrieved 2011-07-30.
- ↑ Keller, Karlheinz (2005). "Photography". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a20_001. ISBN 978-3527306732.
- ↑ Aylmore, M.G; Muir, D.M (2001). "Thiosulfate leaching of gold—A review". Minerals Engineering. 14 (2): 135–174. Bibcode:2001MiEng..14..135A. doi:10.1016/S0892-6875(00)00172-2.
- ↑ McCarty, G. W.; Bremner1, J. M.; Krogmeier1, M. J. (1990). "Evaluation of ammonium thiosulfate as a soil urease inhibitor". Fertilizer Research. 24 (3): 135–139. doi:10.1007/BF01073581. S2CID 28574791.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ↑ Wielgosiński, Grzegorz (2011). "The Reduction of Dioxin Emissions from the Processes of Heat and Power Generation". Journal of the Air & Waste Management Association. 61 (5): 511–526. Bibcode:2011JAWMA..61..511W. doi:10.3155/1047-3289.61.5.511. PMID 21608491. S2CID 44546628.