 | |
| Names | |
|---|---|
| IUPAC name
N-(2,2,6,6-tetramethyl-1-oxopiperidin-1-ium-4-yl)acetamide;tetrafluoroborate | |
| Other names
4-(Acetylamino)-2,2,6,6-tetramethyl-1-oxo-piperidinium tetrafluoroborate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.202.272 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H21BF4N2O2 | |
| Molar mass | 300.10 g·mol−1 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
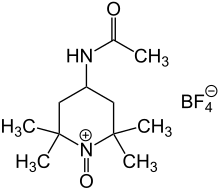
Bobbitt's salt is an oxoammonium compound derived from 4-acetamido-2,2,6,6-tetramethylpiperidine. It contains the tetrafluoroborate anion and is named after the American chemist James M. Bobbitt.
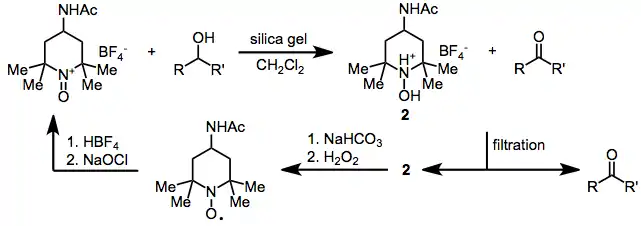
As a less expensive analogue of the N-oxoammonium salt derived from TEMPO, Bobbitt's salt is still mainly used as a catalyst for oxoammonium-catalyzed oxidations.[1][2]
References
- ↑ Nabyl Merbouh; James M. Bobbitt; Christian Brückner (2004). "Preparation of Tetramethylpiperdine-1-oxoammonlum Salts and Their Use as Oxidants in Organic Chemistry. A Review". Organic Preparations and Procedures International. 36: 1-31. doi:10.1080/00304940409355369. S2CID 98117103.
- ↑ James M.Bobbitt; Nicholas A.Eddy; Jay J.Richardson; Stephanie A.Murray; Leon J.Tilley (2013). "Discussion Addendum for: Preparation of 4-Acetylamino-2, 2, 6, 6-tetramethylpiperidine-1-oxoammonium Tetrafluoroborate and the Oxidation of Geraniol to Geranial (2,6-Octadienal, 3,7-dimethyl-, (2e)-)". Org. Synth. 90: 215. doi:10.15227/orgsyn.090.0215.
External links
 Media related to Bobbitt's salt at Wikimedia Commons
Media related to Bobbitt's salt at Wikimedia Commons
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.