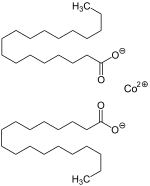
 | |
| Names | |
|---|---|
| Other names
Cobaltous stearate, cobalt distearate, cobalt dioctadecanoate, cobalt(2+) octadecanoate[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.012.449 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C 36H 70CoO 4 | |
| Molar mass | 625.46 |
| Appearance | violet substance |
| Density | 1.7 g/cm3 |
| Melting point | 109 °C (228 °F; 382 K) |
| Boiling point | 359.4 °C (678.9 °F; 632.5 K) |
| insoluble | |
| Hazards | |
| GHS labelling: | |
 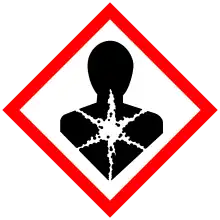 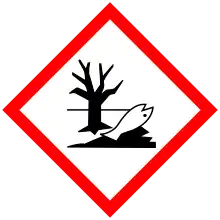 | |
| Danger | |
| H315, H317, H319, H334, H351, H411 | |
| P261, P264, P272, P273, P280, P284, P302+P352, P304+P340, P305+P351+P338, P318, P321, P332+P317, P333+P313, P337+P317, P342+P316, P362+P364, P391, P405, P501 | |
| Flash point | 191 °C (376 °F; 464 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Cobalt(II) stearate is a metal-organic compound, a salt of cobalt and stearic acid with the chemical formula C
36H
70CoO
4.[2][3] The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.[4]
Synthesis
An exchange reaction of sodium stearate and cobalt dichloride:
Physical properties
Cobalt(II) stearate forms a violet substance, occurring in several crystal structures.
Insoluble in water.
Uses
Cobalt(II) stearate is a high-performance bonding agent for rubber. The compound is suitable for applications in natural rubber, cisdene, styrene-butadiene rubber, and their compounds to bond easily with brass- or zinc-plated steel cord or metal plates as well as various bare steel, especially for bonding with brass plating of various thicknesses.[5]
References
- ↑ "CAS 13586-84-0 Cobalt stearate - Alfa Chemistry". Alfa Chemistry. Retrieved 16 February 2023.
- ↑ "Cobalt(II) Stearate". American Elements. Retrieved 16 February 2023.
- ↑ "Cobalt(II) Stearate 1002-88-6 | Tokyo Chemical Industry Co., Ltd.(APAC)". tcichemicals.com. Retrieved 16 February 2023.
- ↑ "Cobalt(II) stearate, Co 9-10%, Thermo Scientific | Fisher Scientific". Fisher Scientific. Retrieved 16 February 2023.
- ↑ "43352 Cobalt(II) stearate, Co 9-10%". Alfa Aesar. Retrieved 16 February 2023.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.