 | |
 | |
| Names | |
|---|---|
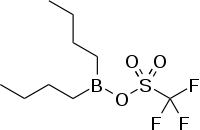
| Preferred IUPAC name
Dibutylborinic trifluoromethanesulfonic anhydride | |
| Other names
Dibutylboron triflate | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | DBBT |
| ChemSpider | |
| ECHA InfoCard | 100.124.520 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H18BF3O3S | |
| Molar mass | 274.11 g·mol−1 |
| Hazards | |
| GHS labelling: | |
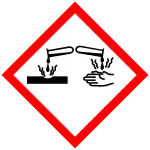 | |
| Danger | |
| H314 | |
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Dibutylboron trifluoromethanesulfonate (also called dibutylboron triflate or DBBT) is a reagent in organic chemistry. Its chemical formula is C9H18BF3O3S. It is used in asymmetric synthesis for example in the formation of boron enolates in the aldol reaction.[1]
References
- ↑ Organic Syntheses, Coll. Vol. 8, p.339 (1993); Vol. 68, p.83 (1990) Link
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.