 | |
| Names | |
|---|---|
| Preferred IUPAC name
Sodium propanoate | |
| Other names
Sodium propionate Napropion E281 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.810 |
| EC Number |
|
| E number | E281 (preservatives) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H5NaO2 | |
| Molar mass | 96.060 g/mol |
| Appearance | Transparent crystals |
| Odor | faint acetic-butyric odor |
| Melting point | 289 °C (552 °F; 562 K) |
| 1 g/ml | |
| Solubility in ethanol | 41.7 g/L |
| Pharmacology | |
| S01AX10 (WHO) QA16QA02 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Sodium propanoate or sodium propionate is the sodium salt of propionic acid which has the chemical formula Na(C2H5COO). This white crystalline solid is deliquescent in moist air.
Reactions
It is produced by the reaction of propionic acid and sodium carbonate or sodium hydroxide.
Uses
It is used as a food preservative and is represented by the food labeling E number E281 in Europe; it is used primarily as a mold inhibitor in bakery products. It is approved for use as a food additive in the EU,[2] USA[3] and Australia and New Zealand[4] (where it is listed by its INS number 281).
Structure
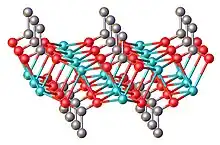
Anhydrous sodium propionate is a polymeric structure, featuring trigonal prismatic Na+ centers bonded to six oxygen ligands provided by the carboxylates. A layered structure is observed, with the hydrophobic ethyl groups projecting into the layered galleries. With hydrated sodium propionate, some of these Na-carboxylate linkages are displaced by water.
See also
- Propionic acid, E 280
- Calcium propionate, E 282
- Potassium propionate, E 283
References
- ↑ Merck Index, 11th Edition, 8623.
- ↑ UK Food Standards Agency: "Current EU approved additives and their E Numbers". Retrieved 2011-10-27.
- ↑ US Food and Drug Administration: "Listing of Food Additives Status Part II". Food and Drug Administration. Retrieved 2011-10-27.
- ↑ Australia New Zealand Food Standards Code"Standard 1.2.4 - Labelling of ingredients". Retrieved 2011-10-27.
- ↑ Fábry, Jan; Samolová, Erika (2020). "Layered alkali propanoatesM+(C2H5COO)−;M+= Na+, K+, Rb+, Cs+". Acta Crystallographica Section E. 76 (9): 1508–1513. doi:10.1107/S2056989020011469. PMC 7472758. PMID 32939309.
External links
- Sodium propanoate at Sci-toys.com