An emergent virus (or emerging virus) is a virus that is either newly appeared, notably increasing in incidence/geographic range or has the potential to increase in the near future.[1] Emergent viruses are a leading cause of emerging infectious diseases and raise public health challenges globally, given their potential to cause outbreaks of disease which can lead to epidemics and pandemics.[2] As well as causing disease, emergent viruses can also have severe economic implications.[3] Recent examples include the SARS-related coronaviruses, which have caused the 2002-2004 outbreak of SARS (SARS-CoV-1) and the 2019–21 pandemic of COVID-19 (SARS-CoV-2).[4][5] Other examples include the human immunodeficiency virus which causes HIV/AIDS; the viruses responsible for Ebola;[6] the H5N1 influenza virus responsible for avian flu;[7] and H1N1/09, which caused the 2009 swine flu pandemic[8] (an earlier emergent strain of H1N1 caused the 1918 Spanish flu pandemic).[9] Viral emergence in humans is often a consequence of zoonosis, which involves a cross-species jump of a viral disease into humans from other animals. As zoonotic viruses exist in animal reservoirs, they are much more difficult to eradicate and can therefore establish persistent infections in human populations.[10]
Emergent viruses should not be confused with re-emerging viruses or newly detected viruses. A re-emerging virus is generally considered to be a previously appeared virus that is experiencing a resurgence,[1][11] for example measles.[12] A newly detected virus is a previously unrecognized virus that had been circulating in the species as endemic or epidemic infections.[13] Newly detected viruses may have escaped classification because they left no distinctive clues, and/or could not be isolated or propagated in cell culture.[14] Examples include human rhinovirus (a leading cause of common colds which was first identified in 1956),[15] hepatitis C (eventually identified in 1989),[16] and human metapneumovirus (first described in 2001, but thought to have been circulating since the 19th century).[17] As the detection of such viruses is technology driven, the number reported is likely to expand.
Zoonosis
Given the rarity of spontaneous development of new virus species, the most frequent cause of emergent viruses in humans is zoonosis. This phenomenon is estimated to account for 73% of all emerging or re-emerging pathogens, with viruses playing a disproportionately large role.[18] RNA viruses are particularly frequent, accounting for 37% of emerging and re-emerging pathogens.[18] A broad range of animals - including wild birds, rodents and bats - are associated with zoonotic viruses.[19] It is not possible to predict specific zoonotic events that may be associated with a particular animal reservoir at any given time.[20]
Zoonotic spillover can either result in self-limited 'dead-end' infections, in which no further human-human transmission occurs (as with the rabies virus),[21] or in infectious cases, in which the zoonotic pathogen is able to sustain human-human transmission (as with the Ebola virus).[6] If the zoonotic virus is able to maintain successful human-human transmission, an outbreak may occur.[22] Some spillover events can also result in the virus adapting exclusively for human infection (as occurred with the HIV virus),[23] in which case humans become a new reservoir for the pathogen.
A successful zoonotic 'jump' depends on human contact with an animal harbouring a virus variant that is able to infect humans. In order to overcome host-range restrictions and sustain efficient human-human transmission, viruses originating from an animal reservoir will normally undergo mutation, genetic recombination and reassortment.[20] Due to their rapid replication and high mutation rates, RNA viruses are more likely to successfully adapt for invasion of a new host population.[3]
Examples of animal sources
Bats

While bats are essential members of many ecosystems,[24] they are also frequently implicated as frequent sources of emerging virus infections.[25] Their immune systems have evolved in such a way as to suppress any inflammatory response to viral infections, thereby allowing them to become tolerant hosts for evolving viruses, and consequently provide major reservoirs of zoonotic viruses.[26] They are associated with more zoonotic viruses per host species than any other mammal, and molecular studies have demonstrated that they are the natural hosts for several high-profile zoonotic viruses, including severe acute respiratory syndrome-related coronaviruses and Ebola/Marburg hemorrhagic fever filoviruses.[27] In terms of their potential for spillover events, bats have taken over the leading role previously assigned to rodents.[26] Viruses can be transmitted from bats via several mechanisms, including bites,[28] aerosolization of saliva (e.g. during echolocation), and faeces/urine.[29]
Due to their distinct ecology/behaviour, bats are naturally more susceptible to viral infection and transmission. Several bat species (e.g. brown bats) aggregate in crowded roosts, which promotes intra- and interspecies viral transmission. Moreover, as bats are widespread in urban areas, humans occasionally encroach on their habitats which are contaminated with guano and urine. Their ability to fly and migration patterns also means that bats are able to spread disease over a large geographic area, while also acquiring new viruses.[30] Additionally, bats experience persistent viral infections which, together with their extreme longevity (some bat species have lifespans of 35 years), helps to maintain viruses and transmit them to other species. Other bat characteristics which contribute to their potency as viral hosts include: their food choices, torpor/hibernation habits, and susceptibility to reinfection.[30]
Drivers of viral emergence
Viral emergence is often a consequence of both nature and human activity. In particular, ecological changes can greatly facilitate the emergence and re-emergence of zoonotic viruses.[31] Factors such as deforestation, reforestation, habitat fragmentation and irrigation can all impact the ways in which humans come into contact with wild animal species, and consequently promote virus emergence.[3][32] In particular, habitat loss of reservoir host species plays a significant role in emerging zoonoses.[33] Additionally, climate change can affect ecosystems and vector distribution, which in turn can affect the emergence of vector-borne viruses. Other ecological changes - for example, species introduction and predator loss - can also affect virus emergence and prevalence. Some agricultural practices, for example livestock intensification and inappropriate management/disposal of farm animal faeces, are also associated with an increased risk of zoonosis.[3][34]
Viruses may also emerge due to the establishment of human populations that are vulnerable to infection. For example, a virus may emerge following loss of cross-protective immunity, which may occur due to loss of a wild virus or termination of vaccination programmes. Well-developed countries also have higher proportions of aging citizens and obesity-related disease, thus meaning that their populations may be more immunosuppressed and therefore at risk of infection.[3] Contrastingly, poorer nations may have immunocompromised populations due to malnutrition or chronic infection; these countries are also unlikely to have stable vaccination programmes.[3] Additionally, changes in human demographics[3] – for example, the birth and/or migration of immunologically naïve individuals – can lead to the development of a susceptible population that enables large-scale virus infection.
Other factors which can promote viral emergence include globalisation; in particular, international trade and human travel/migration can result in the introduction of viruses into new areas.[3] Moreover, as densely populated cities promote rapid pathogen transmission, uncontrolled urbanization (i.e. the increased movement and settling of individuals in urban areas) can promote viral emergence.[35] Animal migration can also lead to the emergence of viruses, as was the case for the West Nile virus which was spread by migrating bird populations.[36] Additionally, human practices regarding food production and consumption can also contribute to the risk of viral emergence. In particular, wet markets (i.e. live animal markets) are an ideal environment for virus transfer, due to the high density of people and wild/farmed animals present.[29] Consumption of bushmeat is also associated with pathogen emergence.[29]
Prevention
Control and prevention of zoonotic diseases depends on appropriate global surveillance at various levels, including identification of novel pathogens, public health surveillance (including serological surveys), and analysis of the risks of transmission.[37] The complexity of zoonotic events around the world predicates a multidisciplinary approach to prevention.[37] The One Health Model has been proposed as a global strategy to help prevent the emergence of zoonotic diseases in humans, including novel viral diseases.[37] The One Health concept aims to promote the health of animals, humans, and the environment, both locally and globally, by fostering understanding and collaboration between practitioners of different interrelated disciplines, including wildlife biology, veterinary science, medicine, agriculture, ecology, microbiology, epidemiology, and biomedical engineering.[37][38]
Virulence of emergent viruses
As hosts are immunologically naïve to pathogens they have not encountered before, emergent viruses are often extremely virulent in terms of their capacity to cause disease. Their high virulence is also due to a lack of adaptation to the new host; viruses normally exert strong selection pressure on the immune systems of their natural hosts, which in turn exerts a strong selection pressure on viruses.[39] This coevolution means that the natural host is able to manage infection. However, when the virus jumps to a new host (e.g. humans), the new host is unable to deal with infection due to a lack of coevolution, which results in mismatch between host immunoeffectors and virus immunomodulators.
Additionally, in order to maximise transmission, viruses often naturally undergo attenuation (i.e. virulence is reduced) so that infected animals can survive long enough to infect other animals more efficiently.[40] However, as attenuation takes time to achieve, new host populations will not initially benefit from this phenomenon. Moreover, as zoonotic viruses also naturally exist in animal reservoirs,[10] their survival is not dependent on transmission between new hosts; this means that emergent viruses are even more unlikely to attenuate for the purpose of maximal transmission, and they remain virulent.
Although emergent viruses are frequently highly virulent, they are limited by several host factors including: innate immunity, natural antibodies and receptor specificity. If the host has previously been infected by a pathogen that is similar to the emergent virus, the host may also benefit from cross-protective immunity.
Examples of emergent viruses
Influenza A
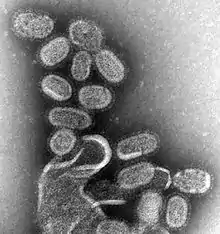
Influenza is a highly contagious respiratory infection, which affects approximately 9% of the global population and causes 300,000 to 500,000 deaths annually.[41][42] Based on their core proteins, influenza viruses are classified into types A, B, C and D.[43][44] While both influenza A and B can cause epidemics in humans, influenza A also has pandemic potential and a higher mutation rate, therefore is most significant to public health.[44][45]
Influenza A viruses are further classified into subtypes, based on the combinations of the surface glycoproteins hemagglutinin (HA) and neuraminidase (NA). The primary natural reservoir for most influenza A subtypes are wild aquatic birds;[44] however, through a series of mutations, a small subset of these viruses have adapted for infection of humans (and other animals).[46] A key determinant of whether a particular influenza A subtype can infect humans is its binding specificity. Avian influenza A preferentially binds to cell surface receptors with a terminal α2,3‐linked sialic acid, while human influenza A preferentially binds to cell surface receptors with a terminal α2,6‐linked sialic acid. Via mutation, some avian influenza A viruses have successfully altered their binding specificity from α2,3‐ to α2,6‐linked sialic acid.[47] However, in order to emerge in humans, avian influenza A viruses must also adapt their RNA polymerases for function in mammalian cells,[48] as well as mutating for stability in the acidic respiratory tract of humans.[49]
Following adaptation and host switch, influenza A viruses have the potential to cause epidemics and pandemics in humans. Minor changes in HA and NA structure (antigenic drift) occur frequently, which enables the virus to cause repetitive outbreaks (i.e. seasonal influenza) by evading immune recognition.[43] Major changes in HA and NA structure (antigenic shift), which are caused by genetic reassortment between different influenza A subtypes (e.g. between human and animal subtypes), can instead cause large regional/global pandemics.[43] Due to the emergence of antigenically different influenza A strains in humans, four pandemics occurred in the 20th century alone.[50]
Additionally, although animal influenza A viruses (e.g. swine influenza) are distinct from human influenza viruses, they can still cause zoonotic infection in humans. These infections are largely acquired following direct contact with infected animals or contaminated environments, but do not result in efficient human-human transmission; examples of this include H5N1 influenza and H7N9 influenza.[44]
SARS-CoV
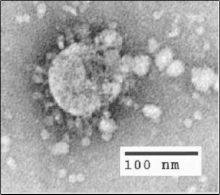
In 2002, a highly pathogenic SARS-CoV (Severe Acute Respiratory Syndrome Coronavirus) strain emerged from a zoonotic reservoir; approximately 8000 people were infected worldwide, and mortality rates approached 50% or more in the elderly.[51] As SARS-CoV is most contagious post-symptoms, the introduction of strict public health measures effectively halted the pandemic.[51] The natural reservoir host for SARS-CoV is thought to be horseshoe bats, although the virus has also been identified in several small carnivores (e.g. palm civets and racoon dogs). The emergence of SARS-CoV is believed to have been facilitated by Chinese wet markets, in which civets positive for the virus acted as intermediate hosts and passed SARS-CoV onto humans (and other species).[51][52] However, more recent analysis suggests that SARS-CoV may have directly jumped from bats to humans, with subsequent cross-transmission between humans and civets.[51]
In order to infect cells, SARS-CoV uses the spike surface glycoprotein to recognise and bind to host ACE-2, which it uses as a cellular entry receptor;[51] the development of this characteristic was crucial in enabling SARS-CoV to ‘jump’ from bats to other species.
MERS-CoV
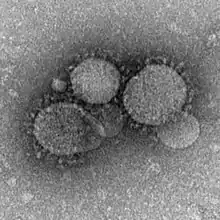
First reported in 2012, MERS-CoV (Middle East Respiratory Syndrome Coronavirus) marks the second known introduction of a highly pathogenic coronavirus from a zoonotic reservoir into humans. The case mortality rate of this emergent virus is approximately 35%, with 80% of all cases reported by Saudi Arabia.[53] Although MERS-CoV is likely to have originated in bats,[54] dromedary camels have been implicated as probable intermediate hosts. MERS-CoV is believed to have been circulating in these mammals for over 20 years,[54] and it is thought that novel camel farming practices drove the spillover of MERS-CoV into humans.[55] Studies have shown that humans can be infected with MERS-CoV via direct or indirect contact within infected dromedary camels, while human-human transmission is limited.[53]
MERS-CoV gains cellular entry by using a spike surface protein to bind to the host DPP4 surface receptor; the core subdomain of this spike surface protein shares similarities with that of SARS-CoV, but its receptor binding subdomain (RBSD) significantly differs.[54]
Bluetongue disease

Bluetongue disease is a non-contagious vector-borne disease caused by bluetongue virus, which affects species of ruminants (particularly sheep).[56] Climate change has been implicated in the emergence and global spread of this disease, due to its impact on vector distribution. The natural vector of the bluetongue virus is the African midge C. imicola, which is normally limited to Africa and subtropical Asia. However, global warming has extended the geographic range of C. imicola, so that it now overlaps with a different vector (C. pulcaris or C. obsoletus) with a much more northward geographic range. This change enabled the bluetongue virus to jump vector, thus causing the northward spread of bluetongue disease into Europe.[57]
See also
References
- 1 2 Holland DJ (February 1998). "Emerging viruses". Current Opinion in Pediatrics. 10 (1): 34–40. doi:10.1097/00008480-199802000-00007. PMID 9529635.
- ↑ Devaux CA (February 2012). "Emerging and re-emerging viruses: A global challenge illustrated by Chikungunya virus outbreaks". World Journal of Virology. 1 (1): 11–22. doi:10.5501/wjv.v1.i1.11. PMC 3782263. PMID 24175207.
- 1 2 3 4 5 6 7 8 Lindahl JF, Grace D (2015). "The consequences of human actions on risks for infectious diseases: a review". Infection Ecology & Epidemiology. 5 (1): 30048. Bibcode:2015InfEE...530048L. doi:10.3402/iee.v5.30048. PMC 4663196. PMID 26615822.
- ↑ Morens DM, Fauci AS (September 2020). "Emerging pandemic diseases: how we got to COVID-19". Cell. 182 (5): 1077–1092. doi:10.1016/j.cell.2020.08.021. PMC 7428724. PMID 32846157.
- ↑ Zheng J (2020). "SARS-CoV-2: an Emerging Coronavirus that Causes a Global Threat". International Journal of Biological Sciences. 16 (10): 1678–1685. doi:10.7150/ijbs.45053. PMC 7098030. PMID 32226285.
- 1 2 Holmes EC, Dudas G, Rambaut A, Andersen KG (October 2016). "The evolution of Ebola virus: Insights from the 2013-2016 epidemic". Nature. 538 (7624): 193–200. Bibcode:2016Natur.538..193H. doi:10.1038/nature19790. PMC 5580494. PMID 27734858.
- ↑ Wei P, Cai Z, Hua J, Yu W, Chen J, Kang K, et al. (2016). "Pains and Gains from China's Experiences with Emerging Epidemics: From SARS to H7N9". BioMed Research International. 2016: 5717108. doi:10.1155/2016/5717108. PMC 4971293. PMID 27525272.
- ↑ Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, et al. (June 2009). "Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic". Nature. 459 (7250): 1122–5. Bibcode:2009Natur.459.1122S. doi:10.1038/nature08182. PMID 19516283.
- ↑ Taubenberger JK, Morens DM (January 2006). "1918 Influenza: the mother of all pandemics". Emerging Infectious Diseases. 12 (1): 15–22. doi:10.3201/eid1201.050979. PMC 3291398. PMID 16494711.
- 1 2 Eidson M. "Zoonotic disease". Britannica. Retrieved 16 April 2020.
- ↑ Miquel Porta, ed. (2008). A Dictionary of Epidemiology. Oxford University Press, USA. p. 78. ISBN 978-0-19-971815-3.
- ↑ Fraser-Bell C (2019). "Global Re-emergence of Measles - 2019 update". Global Biosecurity. 1 (3). doi:10.31646/gbio.43. ISSN 2652-0036.
- ↑ Woolhouse M, Scott F, Hudson Z, Howey R, Chase-Topping M (October 2012). "Human viruses: discovery and emergence". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 367 (1604): 2864–71. doi:10.1098/rstb.2011.0354. PMC 3427559. PMID 22966141.
- ↑ Leland DS, Ginocchio CC (January 2007). "Role of cell culture for virus detection in the age of technology". Clinical Microbiology Reviews. 20 (1): 49–78. doi:10.1128/CMR.00002-06. PMC 1797634. PMID 17223623.
- ↑ Kennedy JL, Turner RB, Braciale T, Heymann PW, Borish L (June 2012). "Pathogenesis of rhinovirus infection". Current Opinion in Virology. 2 (3): 287–93. doi:10.1016/j.coviro.2012.03.008. PMC 3378761. PMID 22542099.
- ↑ Houghton M (November 2009). "The long and winding road leading to the identification of the hepatitis C virus". Journal of Hepatology. 51 (5): 939–48. doi:10.1016/j.jhep.2009.08.004. PMID 19781804.
- ↑ de Graaf M, Osterhaus AD, Fouchier RA, Holmes EC (December 2008). "Evolutionary dynamics of human and avian metapneumoviruses". The Journal of General Virology. 89 (Pt 12): 2933–2942. doi:10.1099/vir.0.2008/006957-0. PMID 19008378.
- 1 2 Woolhouse ME, Gowtage-Sequeria S (December 2005). "Host range and emerging and reemerging pathogens". Emerging Infectious Diseases. 11 (12): 1842–7. doi:10.3201/eid1112.050997. PMC 3367654. PMID 16485468.
- ↑ Kruse H, kirkemo AM, Handeland K (December 2004). "Wildlife as source of zoonotic infections". Emerging Infectious Diseases. 10 (12): 2067–72. doi:10.3201/eid1012.040707. PMC 3323390. PMID 15663840.
- 1 2 Domingo E (2010). "Mechanisms of viral emergence". Veterinary Research. 41 (6): 38. doi:10.1051/vetres/2010010. PMC 2831534. PMID 20167200.
- ↑ Baum SG (2008). "Zoonoses-with friends like this, who needs enemies?". Transactions of the American Clinical and Climatological Association. 119: 39–51, discussion 51–2. PMC 2394705. PMID 18596867.
- ↑ Parrish CR, Holmes EC, Morens DM, Park EC, Burke DS, Calisher CH, et al. (September 2008). "Cross-species virus transmission and the emergence of new epidemic diseases". Microbiology and Molecular Biology Reviews. 72 (3): 457–70. doi:10.1128/MMBR.00004-08. PMC 2546865. PMID 18772285.
- ↑ THE AIDS INSTITUTE. "Where did HIV come from?". THE AIDS INSTITUTE. Retrieved 16 April 2020.
- ↑ National Science Foundation. "The Night Life: Why We Need Bats All the Time-Not Just on Halloween". National Science Foundation. Retrieved 14 April 2020.
- ↑ Shi Z (August 2013). "Emerging infectious diseases associated with bat viruses". Science China Life Sciences. 56 (8): 678–82. doi:10.1007/s11427-013-4517-x. PMC 7088756. PMID 23917838.
- 1 2 Subudhi S, Rapin N, Misra V (2019). "Immune system modulation and viral persistence in bats: understanding viral spillover". Viruses. 11 (2): 192. doi:10.3390/v11020192. PMC 6410205. PMID 30813403.
- ↑ O'Shea TJ, Cryan PM, Cunningham AA, Fooks AR, Hayman DT, Luis AD, et al. (May 2014). "Bat flight and zoonotic viruses". Emerging Infectious Diseases. 20 (5): 741–5. doi:10.3201/eid2005.130539. PMC 4012789. PMID 24750692.
- ↑ Wang LF, Anderson DE (February 2019). "Viruses in bats and potential spillover to animals and humans". Current Opinion in Virology. 34: 79–89. doi:10.1016/j.coviro.2018.12.007. PMC 7102861. PMID 30665189.
- 1 2 3 Kuzmin IV, Bozick B, Guagliardo SA, Kunkel R, Shak JR, Tong S, Rupprecht CE (June 2011). "Bats, emerging infectious diseases, and the rabies paradigm revisited". Emerging Health Threats Journal. 4: 7159. doi:10.3402/ehtj.v4i0.7159. PMC 3168224. PMID 24149032.
- 1 2 Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T (July 2006). "Bats: important reservoir hosts of emerging viruses". Clinical Microbiology Reviews. 19 (3): 531–45. doi:10.1128/CMR.00017-06. PMC 1539106. PMID 16847084.
- ↑ Woolhouse M, Gaunt E (2007). "Ecological origins of novel human pathogens". Critical Reviews in Microbiology. 33 (4): 231–42. doi:10.1080/10408410701647560. PMID 18033594. S2CID 19213392.
- ↑ von Csefalvay C (2023), "Host-vector and multihost systems", Computational Modeling of Infectious Disease, Elsevier, pp. 121–149, doi:10.1016/b978-0-32-395389-4.00013-x, ISBN 978-0-323-95389-4, retrieved 2023-03-02
- ↑ Penakalapati G, Swarthout J, Delahoy MJ, McAliley L, Wodnik B, Levy K, Freeman MC (October 2017). "Exposure to Animal Feces and Human Health: A Systematic Review and Proposed Research Priorities". Environmental Science & Technology. 51 (20): 11537–11552. Bibcode:2017EnST...5111537P. doi:10.1021/acs.est.7b02811. PMC 5647569. PMID 28926696.
- ↑ Neiderud CJ (2015). "How urbanization affects the epidemiology of emerging infectious diseases". Infection Ecology & Epidemiology. 5 (1): 27060. Bibcode:2015InfEE...527060N. doi:10.3402/iee.v5.27060. PMC 4481042. PMID 26112265.
- ↑ Rappole JH, Derrickson SR, Hubálek Z (2000). "Migratory birds and spread of West Nile virus in the Western Hemisphere". Emerging Infectious Diseases. 6 (4): 319–28. doi:10.3201/eid0604.000401. PMC 2640881. PMID 10905964.
- 1 2 3 4 Rahman MT, Sobur MA, Islam MS, et al. (September 2020). "Zoonotic diseases: etiology, impact, and control". Microorganisms. 8 (9): 1405. doi:10.3390/microorganisms8091405. PMC 7563794. PMID 32932606.
- ↑ von Csefalvay C (2023), "Host-vector and multihost systems", Computational Modeling of Infectious Disease, Elsevier, pp. 121–149, doi:10.1016/b978-0-32-395389-4.00013-x, ISBN 978-0-323-95389-4, retrieved 2023-03-02
- ↑ Domínguez-Andrés J, Netea MG (December 2019). "Impact of Historic Migrations and Evolutionary Processes on Human Immunity". Trends in Immunology. 40 (12): 1105–1119. doi:10.1016/j.it.2019.10.001. PMC 7106516. PMID 31786023.
- ↑ Longdon B, Hadfield JD, Day JP, Smith SC, McGonigle JE, Cogni R, et al. (March 2015). "The causes and consequences of changes in virulence following pathogen host shifts". PLOS Pathogens. 11 (3): e1004728. doi:10.1371/journal.ppat.1004728. PMC 4361674. PMID 25774803.
- ↑ Clayville LR (October 2011). "Influenza update: a review of currently available vaccines". P & T. 36 (10): 659–84. PMC 3278149. PMID 22346299.
- ↑ UNICEF. "Influenza". UNICEF. Retrieved 14 April 2020.
- 1 2 3 World Health Organization. "Influenza". World Health Organization. Archived from the original on June 17, 2013. Retrieved 13 April 2020.
- 1 2 3 4 World Health Organization. "Influenza (Avian and other zoonotic)". WHO. Retrieved 13 April 2020.
- ↑ Centers for Disease Control and Prevention (18 November 2019). "Influenza (Flu)". CDC. Retrieved 13 April 2020.
- ↑ Byrd-Leotis L, Cummings RD, Steinhauer DA (July 2017). "The Interplay between the Host Receptor and Influenza Virus Hemagglutinin and Neuraminidase". International Journal of Molecular Sciences. 18 (7): 1541. doi:10.3390/ijms18071541. PMC 5536029. PMID 28714909.
- ↑ Lewis DB (2006). "Avian flu to human influenza". Annual Review of Medicine. 57: 139–54. doi:10.1146/annurev.med.57.121304.131333. PMID 16409141.
- ↑ Long JS, Giotis ES, Moncorgé O, Frise R, Mistry B, James J, et al. (January 2016). "Species difference in ANP32A underlies influenza A virus polymerase host restriction". Nature. 529 (7584): 101–4. Bibcode:2016Natur.529..101L. doi:10.1038/nature16474. PMC 4710677. PMID 26738596.
- ↑ Di Lella S, Herrmann A, Mair CM (June 2016). "Modulation of the pH Stability of Influenza Virus Hemagglutinin: A Host Cell Adaptation Strategy". Biophysical Journal. 110 (11): 2293–2301. Bibcode:2016BpJ...110.2293D. doi:10.1016/j.bpj.2016.04.035. PMC 4906160. PMID 27276248.
- ↑ Alexander DJ (2006). "Avian influenza viruses and human health". Developments in Biologicals. 124: 77–84. PMID 16447497.
- 1 2 3 4 5 Bolles M, Donaldson E, Baric R (December 2011). "SARS-CoV and emergent coronaviruses: viral determinants of interspecies transmission". Current Opinion in Virology. 1 (6): 624–34. doi:10.1016/j.coviro.2011.10.012. PMC 3237677. PMID 22180768.
- ↑ Wang LF, Eaton BT (2007). "Bats, Civets and the Emergence of SARS". Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission. Current Topics in Microbiology and Immunology. Vol. 315. pp. 325–44. doi:10.1007/978-3-540-70962-6_13. ISBN 978-3-540-70961-9. PMC 7120088. PMID 17848070.
- 1 2 WHO. "Middle East respiratory syndrome coronavirus (MERS-CoV)". WHO. Retrieved 15 April 2020.
- 1 2 3 Sharif-Yakan A, Kanj SS (December 2014). "Emergence of MERS-CoV in the Middle East: origins, transmission, treatment, and perspectives". PLOS Pathogens. 10 (12): e1004457. doi:10.1371/journal.ppat.1004457. PMC 4256428. PMID 25474536.
- ↑ Farag E, Sikkema RS, Vinks T, Islam MM, Nour M, Al-Romaihi H, et al. (December 2018). "Drivers of MERS-CoV Emergence in Qatar". Viruses. 11 (1): 22. doi:10.3390/v11010022. PMC 6356962. PMID 30602691.
- ↑ The Center for Food Security and Public Health IS. "Bluetongue" (PDF). CFSPH. Retrieved 14 April 2020.
- ↑ Purse BV, Mellor PS, Rogers DJ, Samuel AR, Mertens PP, Baylis M (February 2005). "Climate change and the recent emergence of bluetongue in Europe". Nature Reviews. Microbiology. 3 (2): 171–81. doi:10.1038/nrmicro1090. PMID 15685226. S2CID 62802662.
Further reading
- Artika IM, Ma'roef CN (May 2017). "Laboratory biosafety for handling emerging viruses". Asian Pacific Journal of Tropical Biomedicine. 7 (5): 483–491. doi:10.1016/j.apjtb.2017.01.020. PMC 7103938. PMID 32289025.
External links
- "Emerging Viruses". MicrobiologyBytes. 2007. Archived from the original on 2007-02-24.
- "National Center for Emerging and Zoonotic Infectious Diseases (NCEZID)". 19 November 2021.