Microvesicles (ectosomes, or microparticles) are a type of extracellular vesicle (EV) that are released from the cell membrane.[1] In multicellular organisms, microvesicles and other EVs are found both in tissues (in the interstitial space between cells) and in many types of body fluids.[2] Delimited by a phospholipid bilayer,[3][4] microvesicles can be as small as the smallest EVs (30 nm in diameter) or as large as 1000 nm. They are considered to be larger, on average, than intracellularly-generated EVs known as exosomes. Microvesicles play a role in intercellular communication and can transport molecules such as mRNA, miRNA, and proteins between cells.[5]
Though initially dismissed as cellular debris, microvesicles may reflect the antigenic content of the cell of origin and have a role in cell signaling. Like other EVs, they have been implicated in numerous physiologic processes, including anti-tumor effects, tumor immune suppression, metastasis, tumor-stroma interactions, angiogenesis, and tissue regeneration.[6][7][8][9] Microvesicles may also remove misfolded proteins, cytotoxic agents and metabolic waste from the cell. Changes in microvesicle levels may indicate diseases including cancer.[10][11]
Formation and contents
Different cells can release microvesicles from the plasma membrane. Sources of microvesicles include megakaryocytes, blood platelets, monocytes, neutrophils, tumor cells and placenta.
Platelets play an important role in maintaining hemostasis: they promote thrombus growth, and thus they prevent loss of blood. Moreover, they enhance immune response, since they express the molecule CD154 (CD40L). Platelets are activated by inflammation, infection, or injury, and after their activation microvesicles containing CD154 are released from platelets. CD154 is a crucial molecule in the development of T cell-dependent humoral immune response. CD154 knockout mice are incapable of producing IgG, IgE, or IgA as a response to antigens. Microvesicles can also transfer prions and molecules CD41 and CXCR4.[12]
Endothelial microparticles
Endothelial microparticles are small vesicles that are released from endothelial cells and can be found circulating in the blood.[13]
The microparticle consists of a plasma membrane surrounding a small amount of cytosol. The membrane of the endothelial microparticle contains receptors and other cell surface molecules which enable the identification of the endothelial origin of the microparticle, and allow it to be distinguished from microparticles from other cells, such as platelets.
Although circulating endothelial microparticles can be found in the blood of normal individuals, increased numbers of circulating endothelial microparticles have been identified in individuals with certain diseases, including hypertension and cardiovascular disorders,[14] and pre-eclampsia[15] and various forms of vasculitis. The endothelial microparticles in some of these disease states have been shown to have arrays of cell surface molecules reflecting a state of endothelial dysfunction. Therefore, endothelial microparticles may be useful as an indicator or index of the functional state of the endothelium in disease, and may potentially play key roles in the pathogenesis of certain diseases, including rheumatoid arthritis.[16]
Endothelial microparticles have been found to prevent apoptosis in recipient cells by inhibiting the p38 pathway via inactivating mitogen-activated protein kinase (MKP)-1. Uptake of endothelial micoparticles is Annexin I/Phosphatidylserine receptor dependant.[17]
Microparticles are derived from many other cell types.[18]
Process of formation
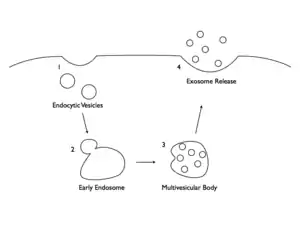
Microvesicles and exosomes are formed and released by two slightly different mechanisms. These processes result in the release of intercellular signaling vesicles. Microvesicles are small, plasma membrane-derived particles that are released into the extracellular environment by the outward budding and fission of the plasma membrane. This budding process involves multiple signaling pathways including the elevation of intracellular calcium and reorganization of the cell's structural scaffolding. The formation and release of microvesicles involve contractile machinery that draws opposing membranes together before pinching off the membrane connection and launching the vesicle into the extracellular space.[19][20][21]
Microvesicle budding takes place at unique locations on the cell membrane that are enriched with specific lipids and proteins reflecting their cellular origin. At these locations, proteins, lipids, and nucleic acids are selectively incorporated into microvesicles and released into the surrounding environment.[20]
Exosomes are membrane-covered vesicles, formed intracellularly are considered to be smaller than 100 nm. In contrast to microvesicles, which are formed through a process of membrane budding, or exocytosis, exosomes are initially formed by endocytosis. Exosomes are formed by invagination within a cell to create an intracellular vesicle called an endosome, or an endocytic vesicle. In general, exosomes are formed by segregating the cargo (e.g., lipids, proteins, and nucleic acids) within the endosome. Once formed, the endosome combines with a structure known as a multivesicular body (MVB). The MVB containing segregated endosomes ultimately fuses with the plasma membrane, resulting in exocytosis of the exosomes.[21][22]
Once formed, both microvesicles and exosomes (collectively called extracellular vesicles) circulate in the extracellular space near the site of release, where they can be taken up by other cells or gradually deteriorate. In addition, some vesicles migrate significant distances by diffusion, ultimately appearing in biological fluids such as cerebrospinal fluid, blood, and urine.[21]
Mechanism of shedding
There are three mechanisms which lead to release of vesicles into the extracellular space. First of these mechanisms is exocytosis from multivesicular bodies and the formation of exosomes. Another mechanism is budding of microvesicles directly from a plasma membrane. And the last one is cell death leading to apoptotic blebbing. These are all energy-requiring processes.
Under physiologic conditions, the plasma membrane of cells has an asymmetric distribution of phospholipids. aminophospholipids, phosphatidylserine, and phosphatidylethanolamine are specifically sequestered in the inner leaflet of the membrane. The transbilayer lipid distribution is under the control of three phospholipidic pumps: an inward-directed pump, or flippase; an outward-directed pump, or floppase; and a lipid scramblase, responsible for non-specific redistribution of lipids across the membrane.
After cell stimulation, including apoptosis, a subsequent cytosolic Ca2+ increase promotes the loss of phospholipid asymmetry of the plasma membrane, subsequent phosphatidylserine exposure, and a transient phospholipidic imbalance between the external leaflet at the expense of the inner leaflet, leading to budding of the plasma membrane and microvesicle release.[23]
Molecular contents
The lipid and protein content of microvesicles has been analyzed using various biochemical techniques. Microvesicles display a spectrum of enclosed molecules enclosed within the vesicles and their plasma membranes. Both the membrane molecular pattern and the internal contents of the vesicle depend on the cellular origin and the molecular processes triggering their formation. Because microvesicles are not intact cells, they do not contain mitochondria, Golgi, endoplasmic reticulum, or a nucleus with its associated DNA.[22][24]
Microvesicle membranes consist mainly of membrane lipids and membrane proteins. Regardless of their cell type of origin, nearly all microvesicles contain proteins involved in membrane transport and fusion. They are surrounded by a phospholipid bilayer composed of several different lipid molecules. The protein content of each microvesicle reflects the origin of the cell from which it was released. For example, those released from antigen-presenting cells (APCs), such as B cells and dendritic cells, are enriched in proteins necessary for adaptive immunity, while microvesicles released from tumors contain proapoptotic molecules and oncogenic receptors (e.g. EGFR).[22]
In addition to the proteins specific to the cell type of origin, some proteins are common to most microvesicles. For example, nearly all contain the cytoplasmic proteins tubulin, actin and actin-binding proteins, as well as many proteins involved in signal transduction, cell structure and motility, and transcription. Most microvesicles contain the so-called "heat-shock proteins" hsp70 and hsp90, which can facilitate interactions with cells of the immune system. Finally, tetraspanin proteins, including CD9, CD37, CD63 and CD81 are one of the most abundant protein families found in microvesicle membranes.[22][24][25][26] Many of these proteins may be involved in the sorting and selection of specific cargos to be loaded into the lumen of the microvesicle or its membrane.[27]
Other than lipids and proteins, microvesicles are enriched with nucleic acids (e.g., messenger RNA (mRNA) and microRNA (miRNA)). The identification of RNA molecules in microvesicles supports the hypothesis that they are a biological vehicle for the transfer of nucleic acids and subsequently modulate the target cell's protein synthesis. Messenger RNA transported from one cell to another through microvesicles can be translated into proteins, conferring new function to the target cell. The discovery that microvesicles may shuttle specific mRNA and miRNA suggests that this may be a new mechanism of genetic exchange between cells.[26][28] Exosomes produced by cells exposed to oxidative stress can mediate protective signals, reducing oxidative stress in recipient cells, a process which is proposed to depend on exosomal RNA transfer.[29] These RNAs are specifically targeted to microvesicles, in some cases containing detectable levels of RNA that is not found in significant amounts in the donor cell.[26]
Because the specific proteins, mRNAs, and miRNAs in microvesicles are highly variable, it is likely that these molecules are specifically packaged into vesicles using an active sorting mechanism. At this point, it is unclear exactly which mechanisms are involved in packaging soluble proteins and nucleic acids into microvesicles.[20][30]
Role on target cells
Once released from their cell of origin, microvesicles interact specifically with cells they recognize by binding to cell-type specific, membrane-bound receptors. Because microvesicles contain a variety of surface molecules, they provide a mechanism for engaging different cell receptors and exchanging material between cells. This interaction ultimately leads to fusion with the target cell and release of the vesicles' components, thereby transferring bioactive molecules, lipids, genetic material, and proteins. The transfer of microvesicle components includes specific mRNAs and proteins, contributing to the proteomic properties of target cells.[26] microvesicles can also transfer miRNAs that are known to regulate gene expression by altering mRNA turnover.[20][21][24][31]
Mechanisms of signaling
Degradation
In some cases, the degradation of microvesicles is necessary for the release of signaling molecules. During microvesicle production, the cell can concentrate and sort the signaling molecules which are released into the extracellular space upon microvesicle degradation. Dendritic cells, macrophage and microglia derived microvesicles contain proinflammatory cytokines and neurons and endothelial cells release growth factors using this mechanism of release.[21]
Fusion
Proteins on the surface of the microvesicle will interact with specific molecules, such as integrin, on the surface of its target cell. Upon binding, the microvesicle can fuse with the plasma membrane. This results in the delivery of nucleotides and soluble proteins into the cytosol of the target cell as well as the integration of lipids and membrane proteins into its plasma membrane.[3]
Internalization
Microvesicles can be endocytosed upon binding to their targets, allowing for additional steps of regulation by the target cell. The microvesicle may fuse, integrating lipids and membrane proteins into the endosome while releasing its contents into the cytoplasm. Alternatively, the endosome may mature into a lysosome causing the degradation of the microvesicle and its contents, in which case the signal is ignored.[3]
Transcytosis
After internalization of microvesicle via endocytosis, the endosome may move across the cell and fuse with the plasma membrane, a process called transcytosis. This results in the ejection of the microvesicle back into the extracellular space or may result in the transportation of the microvesicle into a neighboring cell.[3] This mechanism might explain the ability of microvesicle to cross biological barriers, such as the blood brain barrier, by moving from cell to cell.[32]
Contact dependent signaling
In this form of signaling, the microvesicle does not fuse with the plasma membrane or engulfed by the target cell. Similar to the other mechanisms of signaling, the microvesicle has molecules on its surface that will interact specifically with its target cell. There are additional surface molecules, however, that can interact with receptor molecules which will interact with various signaling pathways.[21] This mechanism of action can be used in processes such as antigen presentation, where MHC molecules on the surface of microvesicle can stimulate an immune response.[27] Alternatively, there may be molecules on microvesicle surfaces that can recruit other proteins to form extracellular protein complexes that may be involved in signaling to the target cell.[21]
Relevance in disease
Cancer
Promoting aggressive tumor phenotypes
The oncogenic receptor ECGFvIII, which is located in a specific type of aggressive glioma tumor, can be transferred to a non-aggressive population of tumor cells via microvesicles. After the oncogenic protein is transferred, the recipient cells become transformed and show characteristic changes in the expression levels of target genes. It is possible that transfer of other mutant oncogenes, such as HER2, may be a general mechanism by which malignant cells cause cancer growth at distant sites.[20][31] Microvesicles from non-cancer cells can signal to cancer cells to become more aggressive. Upon exposure to microvesicles from tumor-associated macrophages, breast cancer cells become more invasive in vitro.[33]
Promoting angiogenesis
Angiogenesis, which is essential for tumor survival and growth, occurs when endothelial cells proliferate to create a matrix of blood vessels that infiltrate the tumor, supplying the nutrients and oxygen necessary for tumor growth. A number of reports have demonstrated that tumor-associated microvesicles release proangiogenic factors that promote endothelial cell proliferation, angiogenesis, and tumor growth. Microvesicles shed by tumor cells and taken up by endothelial cells also facilitate angiogenic effects by transferring specific mRNAs and miRNAs.[21]
Involvement in multidrug resistance
When anticancer drugs such as doxorubicin accumulate in microvesicles, the drug's cellular levels decrease. This can ultimately contribute to the process of drug resistance. Similar processes have been demonstrated in microvesicles released from cisplatin-insensitive cancer cells. Vesicles from these tumors contained nearly three times more cisplatin than those released from cisplatin-sensitive cells. For example, tumor cells can accumulate drugs into microvesicles. Subsequently, the drug-containing microvesicles are released from the cell into the extracellular environment, thereby mediating resistance to chemotherapeutic agents and resulting in significantly increased tumor growth, survival, and metastasis.[20][34]
Interference with antitumor immunity
Microvesicles from various tumor types can express specific cell-surface molecules (e.g. FasL or CD95) that induce T-cell apoptosis and reduce the effectiveness of other immune cells. microvesicles released from lymphoblastoma cells express the immune-suppressing protein latent membrane protein-1 (LMP1), which inhibits T-cell proliferation and prevents the removal of circulating tumor cells (CTCs). As a consequence, tumor cells can turn off T-cell responses or eliminate the antitumor immune cells altogether by releasing microvesicles.[20] the combined use of microvesicles and 5-FU resulted in enhanced chemosensitivity of squamous cell carcinoma cells more than the use of either 5-FU or microvesicle alone[35]
Impact on tumor metastasis
Degradation of the extracellular matrix is a critical step in promoting tumor growth and metastasis. Tumor-derived microvesicles often carry protein-degrading enzymes, including matrix metalloproteinase 2 (MMP-2), MMP-9, and urokinase-type plasminogen activator (uPA). By releasing these proteases, tumor cells can degrade the extracellular matrix and invade surrounding tissues. Likewise, inhibiting MMP-2, MMP-9, and uPA prevents microvesicles from facilitating tumor metastasis. Matrix digestion can also facilitate angiogenesis, which is important for tumor growth and is induced by the horizontal transfer of RNAs from microvesicles.[20]
Cellular Origin of Microvesicles
The release of microvesicles has been shown from endothelial cells, vascular smooth muscle cells, platelets, white blood cells (e.g. leukocytes and lymphocytes), and red blood cells. Although some of these microvesicle populations occur in the blood of healthy individuals and patients, there are obvious changes in number, cellular origin, and composition in various disease states.[36][37] It has become clear that microvesicles play important roles in regulating the cellular processes that lead to disease pathogenesis. Moreover, because microvesicles are released following apoptosis or cell activation, they have the potential to induce or amplify disease processes. Some of the inflammatory and pathological conditions that microvesicles are involved in include cardiovascular disease, hypertension, neurodegenerative disorders, diabetes, and rheumatic diseases.[21][22]
Cardiovascular disease
Microvesicles are involved in cardiovascular disease initiation and progression. Microparticles derived from monocytes aggravate atherosclerosis by modulating inflammatory cells.[38] Additionally, microvesicles can induce clotting by binding to clotting factors or by inducing the expression of clotting factors in other cells.[39] Circulating microvesicles isolated from cardiac surgery patients were found to be thrombogenic in both in vitro assays and in rats. Microvesicles isolated from healthy individuals did not have the same effects and may actually have a role in reducing clotting.[40][39] Tissue factor, an initiator of coagulation, is found in high levels within microvesicles, indicating their role in clotting.[41] Renal mesangial cells exposed to high glucose media release microvesicles containing tissue factor, having an angiogenic effect on endothelial cells.[42]
Inflammation
Microvesicles contain cytokines that can induce inflammation via numerous different pathways.[39] These cells will then release more microvesicles, which have an additive effect. This can call neutrophils and leukocytes to the area, resulting in the aggregation of cells.[3][43] However, microvesicles also seem to be involved in a normal physiological response to disease, as there are increased levels of microvesicles that result from pathology.[39]
Neurological disorders
Microvesicles seem to be involved in a number of neurological diseases. Since they are involved in numerous vascular diseases and inflammation, strokes and multiple sclerosis seem to be other diseases for which microvesicles are involved. Circulating microvesicles seem to have an increased level of phosphorylated tau proteins during early stage Alzheimer's disease. Similarly, increased levels of CD133 are an indicator of epilepsy.[44]
Clinical applications
Detection of cancer
Tumor-associated microvesicles are abundant in the blood, urine, and other body fluids of patients with cancer, and are likely involved in tumor progression. They offer a unique opportunity to noninvasively access the wealth of biological information related to their cells of origin. The quantity and molecular composition of microvesicles released from malignant cells varies considerably compared with those released from normal cells. Thus, the concentration of plasma microvesicles with molecular markers indicative of the disease state may be used as an informative blood-based biosignature for cancer.[19] Microvesicles express many membrane-bound proteins, some of which can be used as tumor biomarkers.[45] Several tumor markers accessible as proteins in blood or urine have been used to screen and diagnose various types of cancer. In general, tumor markers are produced either by the tumor itself or by the body in response to the presence of cancer or some inflammatory conditions. If a tumor marker level is higher than normal, the patient is examined more closely to look for cancer or other conditions. For example, CA19-9, CA-125, and CEA have been used to help diagnose pancreatic, ovarian, and gastrointestinal malignancies, respectively. However, although they have proven clinical utility, none of these tumor markers are highly sensitive or specific. Clinical research data suggest that tumor-specific markers exposed on microvesicles are useful as a clinical tool to diagnose and monitor disease.[46] Research is also ongoing to determine if tumor-specific markers exposed on microvesicles are predictive for therapeutic response.[47][48][49][50]
Evidence produced by independent research groups has demonstrated that microvesicles from the cells of healthy tissues, or selected miRNAs from these microvesicles, can be employed to reverse many tumors in pre-clinical cancer models, and may be used in combination with chemotherapy.[51][52]
Conversely, microvesicles processed from a tumor cell are involved in the transport of cancer proteins and in delivering microRNA to the surrounding healthy tissue. It leads to a change of healthy cell phenotype and creates a tumor-friendly environment. Microvesicles play an important role in tumor angiogenesis and in the degradation of matrix due to the presence of metalloproteases, which facilitate metastasis. They are also involved in intensification of the function of regulatory T-lymphocytes and in the induction of apoptosis of cytotoxic T-lymphocytes, because microvesicles released from a tumor cell contain Fas ligand and TRAIL. They prevent differentiation of monocytes to dendritic cells.
Tumor microvesicles also carry tumor antigen, so they can be an instrument for developing tumor vaccines. Circulating miRNA and segments of DNA in all body fluids can be potential markers for tumor diagnostics.[20]
Microvesicles and Rheumatoid arthritis
Rheumatoid arthritis is a chronic systemic autoimmune disease characterized by inflammation of joints. In the early stage there are abundant Th17 cells producing proinflammatory cytokines IL-17A, IL-17F, TNF, IL-21, and IL-22 in the synovial fluid. regulatory T-lymphocytes have a limited capability to control these cells. In the late stage, the extent of inflammation correlates with numbers of activated macrophages that contribute to joint inflammation and bone and cartilage destruction, because they have the ability to transform themselves into osteoclasts that destroy bone tissue. Synthesis of reactive oxygen species, proteases, and prostaglandins by neutrophils is increased. Activation of platelets via collagen receptor GPVI stimulates the release of microvesicles from platelet cytoplasmic membranes. These microparticles are detectable at a high level in synovial fluid, and they promote joint inflammation by transporting proinflammatory cytokine IL-1.
Biological markers for disease
In addition to detecting cancer, it is possible to use microvesicles as biological markers to give prognoses for various diseases. Many types of neurological diseases are associated with increased level of specific types of circulating microvesicles. For example, elevated levels of phosphorylated tau proteins can be used to diagnose patients in early stages of Alzheimer's. Additionally, it is possible to detect increased levels of CD133 in microvesicles of patients with epilepsy.[44]
Mechanism for drug delivery
Circulating microvesicles may be useful for the delivery of drugs to very specific targets. Using electroporation or centrifugation to insert drugs into microvesicles targeting specific cells, it is possible to target the drug very efficiently.[32] This targeting can help by reducing necessary doses as well as prevent off-target side effects. They can target anti-inflammatory drugs to specific tissues.[43] Additionally, circulating microvesicles can bypass the blood–brain barrier and deliver their cargo to neurons while not having an effect on muscle cells. The blood-brain barrier is typically a difficult obstacle to overcome when designing drugs, and microvesicles may be a means of overcoming it.[32] Current research is looking into efficiently creating microvesicles synthetically, or isolating them from patient or engineered cell lines.[53]
See also
References
- ↑ Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O (2015). "Biological properties of extracellular vesicles and their physiological functions". J Extracell Vesicles. 4: 27066. doi:10.3402/jev.v4.27066. PMC 4433489. PMID 25979354.
- ↑ van der Pol, E.; Böing, A. N.; Gool, E. L.; Nieuwland, R. (1 January 2016). "Recent developments in the nomenclature, presence, isolation, detection and clinical impact of extracellular vesicles". Journal of Thrombosis and Haemostasis. 14 (1): 48–56. doi:10.1111/jth.13190. PMID 26564379.
- 1 2 3 4 5 Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L (November 2010). "Exosomes/microvesicles as a mechanism of cell-to-cell communication". Kidney International. 78 (9): 838–48. doi:10.1038/ki.2010.278. PMID 20703216.
- ↑ van der Pol, E; Böing, AN; Harrison, P; Sturk, A; Nieuwland, R (July 2012). "Classification, functions, and clinical relevance of extracellular vesicles". Pharmacological Reviews. 64 (3): 676–705. doi:10.1124/pr.112.005983. PMID 22722893. S2CID 7764903.
- ↑ Balaj, L.; Lessard, R.; Dai, L.; Cho, Y. J.; Pomeroy, S. L.; Breakefield, X. O.; Skog, J. (2011). "Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences". Nature Communications. 2 (2): 180. Bibcode:2011NatCo...2..180B. doi:10.1038/ncomms1180. PMC 3040683. PMID 21285958.
- ↑ Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M. Z. (2006). "Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery". Leukemia. 20 (5): 847–856. doi:10.1038/sj.leu.2404132. PMID 16453000.
- ↑ Hunter, M.; Ismail, N.; Zhang, X.; Aguda, B.; Lee, E.; Yu, L.; Xiao, T.; Schafer, J.; Lee, M.; Schmittgen, T. D.; Nana-Sinkam, S. P.; Jarjoura, D.; Marsh, C. B. (2008). Lo, Yuk Ming Dennis (ed.). "Detection of microRNA Expression in Human Peripheral Blood Microvesicles". PLOS ONE. 3 (11): e3694. Bibcode:2008PLoSO...3.3694H. doi:10.1371/journal.pone.0003694. PMC 2577891. PMID 19002258.
- ↑ Aliotta, J.; Pereira, M.; Johnson, K.; De Paz, N.; Dooner, M.; Puente, N.; Ayala, C.; Brilliant, K.; Berz, D.; Lee, D.; Ramratnam, B.; McMillan, P. N.; Hixson, D. C.; Josic, D.; Quesenberry, P. J. (2010). "Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription". Experimental Hematology. 38 (3): 233–245. doi:10.1016/j.exphem.2010.01.002. PMC 2829939. PMID 20079801.
- ↑ Castellana, D.; Zobairi, F.; Martinez, M. C.; Panaro, M. A.; Mitolo, V.; Freyssinet, J. -M.; Kunzelmann, C. (2009). "Membrane Microvesicles as Actors in the Establishment of a Favorable Prostatic Tumoral Niche: A Role for Activated Fibroblasts and CX3CL1-CX3CR1 Axis". Cancer Research. 69 (3): 785–793. doi:10.1158/0008-5472.CAN-08-1946. PMID 19155311.
- ↑ Dhondt, Bert; Rousseau, Quentin; De Wever, Olivier; Hendrix, An (2016-06-11). "Function of extracellular vesicle-associated miRNAs in metastasis". Cell and Tissue Research. 365 (3): 621–641. doi:10.1007/s00441-016-2430-x. hdl:1854/LU-7250365. ISSN 0302-766X. PMID 27289232. S2CID 2746182.
- ↑ Williams, C.; Royo, F.; Aizpurua-Olaizola, O.; Pazos, R.; Boons, G-J.; Reichardt, N-C.; Falcon-Perez, J.M. (2018). "Glycosylation of extracellular vesicles: current knowledge, tools and clinical perspectives". Journal of Extracellular Vesicles. 7 (1): 1442985. doi:10.1080/20013078.2018.1442985. PMC 5844028. PMID 29535851.
- ↑ Sprague DL, Elzey BD, Crist SA, Waldschmidt TJ, Jensen RJ, Ratliff TL (May 2008). "Platelet-mediated modulation of adaptive immunity: unique delivery of CD154 signal by platelet-derived membrane vesicles". Blood. 111 (10): 5028–36. doi:10.1182/blood-2007-06-097410. PMC 2384131. PMID 18198347.
- ↑ Davizon, Pavela; López, José (September 2009). "Microparticles and thrombotic disease". Current Opinion in Hematology. 16 (5): 334–341. doi:10.1097/MOH.0b013e32832ea49c. PMID 19606028. S2CID 8442260.
- ↑ Boulanger, Chantal M (March 2010). "Microparticles, vascular function and hypertension". Current Opinion in Nephrology and Hypertension. 19 (2): 177–180. doi:10.1097/MNH.0b013e32833640fd. PMID 20051854. S2CID 38211873.
- ↑ Ling L (Feb 2014). "Evaluation of plasma endothelial microparticles in pre-eclampsia". J Int Med Res. 42 (1): 42–51. doi:10.1177/0300060513504362. PMID 24319051.
- ↑ Boilard, E.; et al. (January 2010). "Platelets Amplify Inflammation in Arthritis via Collagen-Dependent Microparticle Production". Science. 327 (5965): 580–583. Bibcode:2010Sci...327..580B. doi:10.1126/science.1181928. PMC 2927861. PMID 20110505.
- ↑ Jansen, Felix; Yang, Xiaoyan; Hoyer, Friedrich Felix; Paul, Kathrin; Heiermann, Nadine; Becher, Marc Ulrich; Hussein, Nebal Abu; Kebschull, Moritz; Bedorf, Jörg; Franklin, Bernardo S.; Latz, Eicke; Nickenig, Georg; Werner, Nikos (14 Jun 2012). "Endothelial Microparticle Uptake in Target Cells Is Annexin I/Phosphatidylserine Receptor Dependent and Prevents Apoptosis". Arteriosclerosis, Thrombosis, and Vascular Biology. 32 (8): 1925–1935. doi:10.1161/ATVBAHA.112.253229. ISSN 1079-5642. PMID 22701020. S2CID 226320.
- ↑ Burnouf, T (October 2015). "An overview of the role of microparticles/microvesicles in blood components: Are they clinically beneficial or harmful?". Transfus Apher Sci. 53 (2): 137–45. doi:10.1016/j.transci.2015.10.010. PMID 26596959.
- 1 2 Van Doormaal, FF; Kleinjan, A; Di Nisio, M; Büller, HR; Nieuwland, R (2009). "Cell-derived microvesicles and cancer". The Netherlands Journal of Medicine. 67 (7): 266–73. PMID 19687520.
- 1 2 3 4 5 6 7 8 9 Muralidharan-Chari V, Clancy JW, Sedgwick A, D'Souza-Schorey C (May 2010). "Microvesicles: mediators of extracellular communication during cancer progression". Journal of Cell Science. 123 (Pt 10): 1603–11. doi:10.1242/jcs.064386. PMC 2864708. PMID 20445011.
- 1 2 3 4 5 6 7 8 9 Cocucci, Emanuele; Racchetti, Gabriella; Meldolesi, Jacopo (2009). "Shedding microvesicles: artefacts no more". Trends in Cell Biology. 19 (2): 43–51. doi:10.1016/j.tcb.2008.11.003. PMID 19144520.
- 1 2 3 4 5 Pap, E.; Pállinger, É.; Pásztói, M.; Falus, A. (2009). "Highlights of a new type of intercellular communication: microvesicle-based information transfer". Inflammation Research. 58 (1): 1–8. doi:10.1007/s00011-008-8210-7. PMID 19132498. S2CID 23475443.
- ↑ Hugel, B.; Martinez, M. C.; Kunzelmann, C.; Freyssinet, J. -M. (2005). "Membrane Microparticles: Two Sides of the Coin". Physiology. 20: 22–27. doi:10.1152/physiol.00029.2004. PMID 15653836. S2CID 20507534.
- 1 2 3 Schorey, Jeffrey S.; Bhatnagar, Sanchita (2008). "Exosome Function: From Tumor Immunology to Pathogen Biology". Traffic. 9 (6): 871–81. doi:10.1111/j.1600-0854.2008.00734.x. PMC 3636814. PMID 18331451.
- ↑ Simpson, Richard J.; Jensen, Søren S.; Lim, Justin W. E. (2008). "Proteomic profiling of exosomes: Current perspectives". Proteomics. 8 (19): 4083–99. doi:10.1002/pmic.200800109. PMID 18780348. S2CID 660825.
- 1 2 3 4 Valadi, Hadi; Ekström, Karin; Bossios, Apostolos; Sjöstrand, Margareta; Lee, James J; Lötvall, Jan O (2007). "Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells". Nature Cell Biology. 9 (6): 654–9. doi:10.1038/ncb1596. PMID 17486113. S2CID 8599814.
- 1 2 Raposo, G; Stoorvogel, W (Feb 18, 2013). "Extracellular vesicles: Exosomes, microvesicles, and friends". The Journal of Cell Biology. 200 (4): 373–83. doi:10.1083/jcb.201211138. PMC 3575529. PMID 23420871.
- ↑ Lewin, Alfred; Yuan, Alex; Farber, Erica L.; Rapoport, Ana Lia; Tejada, Desiree; Deniskin, Roman; Akhmedov, Novrouz B.; Farber, Debora B. (2009). Lewin, Alfred (ed.). "Transfer of MicroRNAs by Embryonic Stem Cell Microvesicles". PLOS ONE. 4 (3): e4722. Bibcode:2009PLoSO...4.4722Y. doi:10.1371/journal.pone.0004722. PMC 2648987. PMID 19266099.
- ↑ Eldh M, Ekström K, Valadi H, Sjöstrand M, Olsson B, Jernås M, Lötvall J. Exosomes Communicate Protective Messages during Oxidative Stress; Possible Role of Exosomal Shuttle RNA. PLoS One. 2010 Dec 17;5(12):e15353.
- ↑ Simons, Mikael; Raposo, Graça (2009). "Exosomes – vesicular carriers for intercellular communication". Current Opinion in Cell Biology. 21 (4): 575–81. doi:10.1016/j.ceb.2009.03.007. PMID 19442504.
- 1 2 Ratajczak, J; Miekus, K; Kucia, M; Zhang, J; Reca, R; Dvorak, P; Ratajczak, M Z (2006). "Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery". Leukemia. 20 (5): 847–56. doi:10.1038/sj.leu.2404132. PMID 16453000.
- 1 2 3 Lakhal, S; Wood, MJ (October 2011). "Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers". BioEssays. 33 (10): 737–41. doi:10.1002/bies.201100076. PMID 21932222. S2CID 20386810.
- ↑ Yang, M; Chen, J; Su, F; Yu, B; Su, F; Lin, L; Liu, Y; Huang, JD; Song, E (Sep 22, 2011). "Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells". Molecular Cancer. 10: 117. doi:10.1186/1476-4598-10-117. PMC 3190352. PMID 21939504.
- ↑ Shedden, Kerby; Xie, Xue Tao; Chandaroy, Parthapratim; Chang, Young Tae; Rosania, Gustavo R. (2003). "Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles". Cancer Research. 63 (15): 4331–7. PMID 12907600.
- ↑ Ghada A. Abd El Latif , Iman M. Aboushady and Dina Sabry Decreased VEGF and cyclin D1 genes expression enhances chemosensitivity of human squamous cell carcinoma cells to 5-fluorouracil and/or mesenchymal stem cells-derived microvesicles E.D.J. Vol. 65, 2, Pp 1217-1228 ; 2019. DOI: 10.21608/EDJ.2019.72197
- ↑ Nieuwland, R (2012). Platelet-Derived Microparticles. San Diego, CA: Academic Press. pp. 453–67. ISBN 978-0123878373.
- ↑ Vanwijk, M; Vanbavel, E; Sturk, A; Nieuwland, R (2003). "Microparticles in cardiovascular diseases". Cardiovascular Research. 59 (2): 277–87. doi:10.1016/S0008-6363(03)00367-5. PMID 12909311.
- ↑ Hoyer, Friedrich Felix; Giesen, Meike Kristin; Nunes França, Carolina; Lütjohann, Dieter; Nickenig, Georg; Werner, Nikos (November 2012). "Monocytic microparticles promote atherogenesis by modulating inflammatory cells in mice". Journal of Cellular and Molecular Medicine. 16 (11): 2777–2788. doi:10.1111/j.1582-4934.2012.01595.x. PMC 4118246. PMID 22697268.
- 1 2 3 4 Distler, JH; Pisetsky, DS; Huber, LC; Kalden, JR; Gay, S; Distler, O (November 2005). "Microparticles as regulators of inflammation: novel players of cellular crosstalk in the rheumatic diseases". Arthritis and Rheumatism. 52 (11): 3337–48. doi:10.1002/art.21350. PMID 16255015.
- ↑ Biró, E; Sturk-Maquelin, KN; Vogel, GM; Meuleman, DG; Smit, MJ; Hack, CE; Sturk, A; Nieuwland, R (December 2003). "Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner". Journal of Thrombosis and Haemostasis. 1 (12): 2561–8. doi:10.1046/j.1538-7836.2003.00456.x. PMID 14738565. S2CID 22275556.
- ↑ Müller, I; Klocke, A; Alex, M; Kotzsch, M; Luther, T; Morgenstern, E; Zieseniss, S; Zahler, S; Preissner, K; Engelmann, B (March 2003). "Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets" (PDF). FASEB Journal. 17 (3): 476–78. doi:10.1096/fj.02-0574fje. PMID 12514112. S2CID 18415836.
- ↑ Shai, E; Varon, D (January 2011). "Development, cell differentiation, angiogenesis--microparticles and their roles in angiogenesis". Arteriosclerosis, Thrombosis, and Vascular Biology. 31 (1): 10–4. doi:10.1161/atvbaha.109.200980. PMID 21160063. S2CID 207728332.
- 1 2 Sun, D; Zhuang, X; Xiang, X; Liu, Y; Zhang, S; Liu, C; Barnes, S; Grizzle, W; Miller, D; Zhang, HG (September 2010). "A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes". Molecular Therapy. 18 (9): 1606–14. doi:10.1038/mt.2010.105. PMC 2956928. PMID 20571541.
- 1 2 Colombo, E; Borgiani, B; Verderio, C; Furlan, R (2012). "Microvesicles: novel biomarkers for neurological disorders". Frontiers in Physiology. 3: 63. doi:10.3389/fphys.2012.00063. PMC 3315111. PMID 22479250.
- ↑ Dhondt, Bert; Geeurickx, Edward; Tulkens, Joeri; Van Deun, Jan; Vergauwen, Glenn; Lippens, Lien; Miinalainen, Ilkka; Rappu, Pekka; Heino, Jyrki; Ost, Piet; Lumen, Nicolaas; De Wever, Olivier; Hendrix, An (11 March 2020). "Unravelling the proteomic landscape of extracellular vesicles in prostate cancer by density-based fractionation of urine". Journal of Extracellular Vesicles. 9 (1): 1736935. doi:10.1080/20013078.2020.1736935. PMC 7144211. PMID 32284825.
- ↑ Dhondt, Bert; Van Deun, Jan; Vermaerke, Silke; de Marco, Ario; Lumen, Nicolaas; De Wever, Olivier; Hendrix, An (June 2018). "Urinary extracellular vesicle biomarkers in urological cancers: From discovery towards clinical implementation". The International Journal of Biochemistry & Cell Biology. 99: 236–256. doi:10.1016/j.biocel.2018.04.009. ISSN 1357-2725. PMID 29654900. S2CID 4876604.
- ↑ Larkin, Samantha ET; Zeidan, Bashar; Taylor, Matthew G; Bickers, Bridget; Al-Ruwaili, Jamal; Aukim-Hastie, Claire; Townsend, Paul A (2010). "Proteomics in prostate cancer biomarker discovery". Expert Review of Proteomics. 7 (1): 93–102. doi:10.1586/epr.09.89. PMID 20121479. S2CID 29689761.
- ↑ Pawlowski, Traci L.; Spetzler, David; Tinder, Teresa; Esmay, Paula; Conrad, Amber; Ellis, Phil; Kennedy, Patrick; Tyrell, Annemarie; et al. (April 20, 2010). Identifying and characterizing subpopulation of exosomes to provide the foundation for a novel exosome-based cancer diagnostic platform. Proceedings of the 101st Annual Meeting of the American Association for Cancer Research.
- ↑ Kuslich, Christine; Pawlowski, Traci L.; Deng, Ta; Tinder, Teresa; Kim, Joon; Kimbrough, Jeff; Spetzler, David (2010). A Sensitive exosome-based biosignature for the diagnosis of prostate cancer (PDF). Proceedings of the 2010 American Society of Clinical Oncology Annual Meeting. Archived from the original (PDF) on 2011-07-10. Also published as Kuslich, Christine; Pawlowski, Traci L.; Deng, Ta; Tinder, Teresa; Kim, Joon; Kimbrough, Jeff; Spetzler, David (May 2010). "A sensitive exosome-based biosignature for the diagnosis of prostate cancer". Journal of Clinical Oncology. 28 (15 suppl): 4636. doi:10.1200/jco.2010.28.15_suppl.4636.
- ↑ Kuslich, Christine; Pawlowski, Traci; Kimbrough, Jeff; Deng, Ta; Tinder, Teresa; Kim, Joon; Spetzler, David (April 18, 2010). Plasma exosomes are a robust biosignature for prostate cancer. Proceedings of the 101st Annual Meeting of the American Association for Cancer Research. Also published as Kuslich, Christine; Pawlowski, Traci; Kimbrough, Jeff; Deng, Ta; Tinder, Teresa; Kim, Joon; Spetzler, David (2010). "Circulating exosomes are a robust biosignature for prostate cancer" (PDF). Caris Life Sciences. Archived from the original (PDF) on 2016-03-04. Retrieved 2017-11-07.
- ↑ Microvesicles (MVS) Derived From Adult Stem Cells For Use In The Therapeutic Treatment of a Tumor Disease. PCT/EP2011/052945 Available online
- ↑ Human Liver Stem Cell-Derived Microvesicles Inhibit Hepatoma Growth in SCID Mice by Delivering Antitumor MicroRNAs. Camussi et al; Stem Cells [2012,30]Available online
- ↑ "CORDIS | European Commission". Archived from the original on 2008-03-28. Retrieved 2017-11-07.
Further reading
- Nilsson, J; Skog, J; Nordstrand, A; Baranov, V; Mincheva-Nilsson, L; Breakefield, X O; Widmark, A (2009). "Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer". British Journal of Cancer. 100 (10): 1–5. doi:10.1038/sj.bjc.6605058. PMC 2696767. PMID 19401683.
- Al-Nedawi, Khalid; Meehan, Brian; Rak, Janusz (2009). "Microvesicles: messengers and mediators of tumor progression". Cell Cycle. 8 (13): 2014–8. doi:10.4161/cc.8.13.8988. PMID 19535896.