| "Candidatus Epulopiscium" | |
|---|---|
| Scientific classification | |
| Domain: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | "Candidatus Epulopiscium" corrig. Montgomery and Pollak 1988 |
| Binomial name | |
| "Candidatus Epulopiscium fishelsonii" corrig. Montgomery and Pollak 1988 | |
| Type strain | |
| USNM 40601 | |
| Synonyms | |
| |
Candidatus Epulopiscium is a genus of Gram-positive bacteria that have a symbiotic relationship with surgeonfish. These bacteria are known for their unusually large size, many ranging from 0.2 - 0.7 mm (200–700 μm) in length. Until the discovery of Thiomargarita namibiensis in 1999, Epulonipiscium species were thought to be the largest bacteria.[1][2] They are still the largest known heterotrophic bacteria.
In addition to their large size, Epulonipiscium, commonly referred to as "epulos," are morphologically diverse and extremely polyploid.[3] Epulos also have unique reproductive strategies in which certain cells can form intracellular offspring, similar to microbial sporulation; furthermore, several epulo morphologies exhibit sporulation.
While the bacteria have not been successfully grown in the lab, scientists have gained a better understanding of Epulonipiscium through microscopic, phylogenetic, and genomic analyses.
Naming and discovery
Epulonipiscium means "a guest at a banquet of fish" in Latin, from epulonum ("guest at a feast" or "guest at a banquet") and piscium ("of a fish"),[4] as the organism was found inside the gut of marine surgeonfish. Epulonipiscium cells were initially classified as protists on the basis of their large size and unusual ultrastructure.
Originally, Epulonipiscium populations were thought to be a single species and given the name Epulopiscium fishelsoni in 1988, by Montgomery (one of the co-discovers) and Pollak. The epithet fishelsoni honors Lev Fishelson, a Polish-born Israeli ichthyologist[5][6] who was part of the group that made the discovery while studying the intestines of a brown surgeonfish from the Red Sea in 1985.[7]
Later, however, Epulopiscium fishelsoni was shown to comprise two phylogenetically distinct groups of bacteria by Angert and collaborators using rRNA gene sequence comparisons.[1] Subsequent studies illustrated the relationship between these symbionts and the host surgeonfish.
Physiology
The largest Epulonipiscium cells can be seen with the naked eye. However, because of their size, Epulonipiscium cells must compensate for their small surface-to-volume ratio, compared to other bacteria. One distinct feature is the cell membrane, which contains many folds to increase the effective surface area.
Additionally, Epulonipiscium cells are extremely polyploid, with individuals containing hundreds of thousands of copies of the genome. Since bacteria rely on diffusion rather than cytoskeletal transport as in eukaryotes, this extreme polyploidy allows for the production of gene products at numerous sites in the cell to produce biomolecules where they are needed.
Reproduction
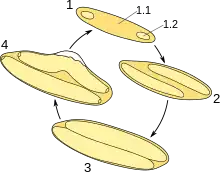
The largest Epulonipiscium morphologies exhibit a unique viviparous reproduction. This unusual and derived form of sporulation produces anywhere from one to twelve daughter cells that grow inside of the parent cell, until the parent eventually lyses, and dies.[9][10] These cells appear to not use binary fission for reproduction. Some morphologies use endospore formation for reproduction.[11] However, there are some smaller morphologies that reproduce through binary fission and spore formation.
Although sporulation is widespread among other bacteria (such as Bacillus subtilis and Clostridium species) in the phylum Bacillota, spore formation is usually brought about by overcrowding, the accumulation of toxins in the environment, or starvation, rather than a standard form of reproduction. The production of multiple endospores has been observed in other large gut symbionts such as Metabacterium polyspora, which are phylogenetically related to Epulonipiscium.[2] Since sporulation affords bacteria much more protection from the outside environment than binary fission, it is thought that the evolution of this unusual life cycle may assist transfer of the bacteria from one host to another.
Symbiosis
Epulonipiscium species and their surgeonfish hosts are suggested to have a nutritional symbiotic relationship: Epulonipiscium species have only been found in surgeonfish that eat algae and detritus. It is suggested that Epulonipiscium species assist in the fish's digestion.[12] However, scientists have been unable to culture Epulonipiscium outside of its natural habitat.
References
- 1 2 Angert ER, Clements KD, Pace NR (March 1993). "The largest bacterium". Nature. 362 (6417): 239–241. Bibcode:1993Natur.362..239A. doi:10.1038/362239a0. PMID 8459849. S2CID 4242187.
- 1 2 Angert ER, Brooks AE, Pace NR (March 1996). "Phylogenetic analysis of Metabacterium polyspora: clues to the evolutionary origin of daughter cell production in Epulopiscium species, the largest bacteria". Journal of Bacteriology. 178 (5): 1451–1456. doi:10.1128/jb.178.5.1451-1456.1996. PMC 177821. PMID 8631724.
- ↑ Hutchison E, Yager NA, Taw MN, Taylor M, Arroyo F, Sannino DR, Angert ER (January 2018). "Developmental stage influences chromosome segregation patterns and arrangement in the extremely polyploid, giant bacterium Epulopiscium sp. type B". Molecular Microbiology. 107 (1): 68–80. doi:10.1111/mmi.13860. PMID 29024073.
- ↑ Prescott LM, Sherwood LM, Woolverton CJ (2006). "Procaryotic Cell Structureand Function" (PDF). Microbiology. p. 43.
- ↑ Goren M (2013). "Professor Lev Fishelson, renowned and respected biologist 1923-2013". Israel Journal of Ecology & Evolution. 59 (3): 164. doi:10.1080/15659801.2013.899808.
- ↑ "Professor Emeritus, Lev Fishelson". Dept. of Zoology, Faculty of Life Sciences. Tel Aviv University.
- ↑ Fishelson, Lev; Montgomery, W. Linn; Myrberg, Arthur A. (July 1985). "A unique symbiosis in the gut of tropical herbivorous surgeonfish (acanthuridae: teleostei) from the red sea". Science. 229 (4708): 49–51. Bibcode:1985Sci...229...49F. doi:10.1126/science.229.4708.49. PMID 17795131.
- ↑ Mendell JE, Clements KD, Choat JH, Angert ER (May 2008). "Extreme polyploidy in a large bacterium". Proceedings of the National Academy of Sciences of the United States of America. 105 (18): 6730–6734. doi:10.1073/pnas.0707522105. PMC 2373351. PMID 18445653.
- ↑ Angert ER, Clements KD (February 2004). "Initiation of intracellular offspring in Epulopiscium". Molecular Microbiology. 51 (3): 827–835. doi:10.1046/j.1365-2958.2003.03869.x. PMID 14731282. S2CID 19426788.
- ↑ "Binary Fission and other Forms of Reproduction in Bacteria: Intracellular offspring production by some Firmicutes". Epulopiscium Website. Cornell Dept. of Microbiology.
- ↑ Angert ER (March 2005). "Alternatives to binary fission in bacteria". Nature Reviews. Microbiology. 3 (3): 214–224. doi:10.1038/nrmicro1096. PMID 15738949. S2CID 8295873.
- ↑ Pollak PE, Montgomery WL (1994-08-01). "Giant bacterium (Epulopiscium fishelsoni) influences digestive enzyme activity of an herbivorous surgeonfish (Acanthurus nigrofuscus)". Comparative Biochemistry and Physiology Part A: Physiology. 108 (4): 657–662. doi:10.1016/0300-9629(94)90352-2. ISSN 0300-9629.
Further reading
- Flint JF, Drzymalski D, Montgomery WL, Southam G, Angert ER (November 2005). "Nocturnal production of endospores in natural populations of epulopiscium-like surgeonfish symbionts". Journal of Bacteriology. 187 (21): 7460–7470. doi:10.1128/JB.187.21.7460-7470.2005. PMC 1272977. PMID 16237029.
- Robinow C, Angert ER (October 1998). "Nucleoids and coated vesicles of "Epulopiscium" spp". Archives of Microbiology. 170 (4): 227–235. doi:10.1007/s002030050637. PMID 9732436. S2CID 6170628.
External links
- Epulonipiscium species and related surgeonfish symbionts, Department of Microbiology, Cornell University. (Accessed May 2014.)
- "Researchers study bacterium big enough to see—the Shaquille O'Neal of bacteria". Physorg.com. 2008-05-07. Retrieved 2008-05-10.