 | |
| Names | |
|---|---|
| IUPAC name
(2S,3R,4E)-3-Hydroxy-2-octadecanamidooctadec-4-en-1-yl (5-acetamido-3,5-dideoxy-D-glycero-α-D-galacto-non-2-ulopyranosylonic acid)-(2→3)-[2-acetamido-2-deoxy-β-D-galactopyranosyl-(1→4)]-β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside | |
| Systematic IUPAC name
(2S,4S,5R,6R)-5-Acetamido-2-{[(2R,3S,4R,5R,6S)-3-{[(2S,3R,4R,5R,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-6-{[(2R,3S,4R,5R,6R)-4,5-dihydroxy-2-(hydroxymethyl)-6-{[(2S,3R,4E)-3-hydroxy-2-octadecanamidooctadec-4-en-1-yl]oxy}oxan-3-yl]oxy}-5-hydroxy-2-(hydroxymethyl)oxan-4-yl]oxy}-4-hydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylate | |
| Other names
Ganglioside GM2; Tay–Sachs ganglioside; β-D-GalNAc-(1→4)-[α-Neu5Ac-(2→3)]-β-D-Gal-(1→4)-β-D-Glc-(1↔1)-N-octadecanoylsphingosine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C67H121N3O26 | |
| Molar mass | 1384.700 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
In organic chemistry, GM2 is a type of ganglioside. G refers to ganglioside, the M is for monosialic (as in it has one sialic acid), and 2 refers to the fact that it was the second monosialic ganglioside discovered. It is associated with GM2 gangliosidoses such as Tay–Sachs disease.[1]
See also
Additional images
 Sphingolipidoses
Sphingolipidoses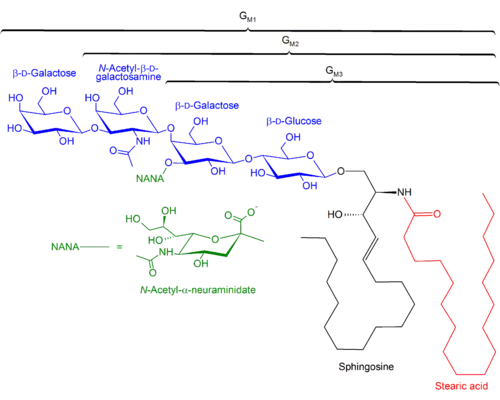 Structures of GM1, GM2, GM3 gangliosides
Structures of GM1, GM2, GM3 gangliosides
References
External links
- Ganglioside+GM2 at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.