 | |
| Names | |
|---|---|
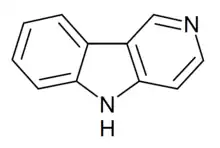
| Preferred IUPAC name
5H-Pyrido[4,3-b]indole | |
| Other names
5H-γ-Carboline | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H8N2 | |
| Molar mass | 168.20 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
γ-Carboline (5H-pyrido[4,3-b]indole), is a nitrogen containing heterocycle. A large number of derivatives are known with varying pharmacological properties.[1][2][3][4]
See also
References
- ↑ Alekseyev RS, Kurki AV, Yurovskaya MA (2009). "γ-Carbolines and their hydrogenated derivatives. 1. Aromatic γ-carbolines: methods of synthesis, chemical and biological properties (review)". Chem Heterocycl Comp. 45 (8): 889–925. doi:10.1007/s10593-009-0373-9. S2CID 97096571.
- ↑ Alekseyev RS, Kurkin AV, Yurovskaya MA (2011). "γ-Carbolines and their hydrogenated derivatives 3. Hydrogenated derivatives of γ-carbolines: chemical and biological properties (Review)". Chem Heterocycl Comp. 46 (10): 1169–1198. doi:10.1007/s10593-011-0652-0. S2CID 95229099.
- ↑ Smirnova OB, Golovko TV, Granik VG (2011). "Carbolines. Part I: Comparison of some methods for the synthesis of α-, γ-, and δ-carbolines (a review)". Pharm Chem J. 44 (12): 654–678. doi:10.1007/s11094-011-0540-z. S2CID 7100718.
- ↑ Smirnova OB, Golovko TV, Granik VG (2011). "Carbolines. Part 2: Comparison of some of the properties of α-, γ-, and δ-carbolines (Review)". Pharm Chem J. 45 (7): 389–400. doi:10.1007/s11094-011-0641-8. S2CID 26600701.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.