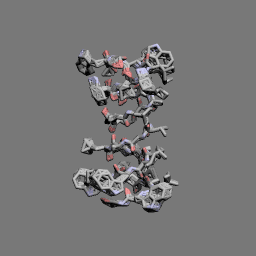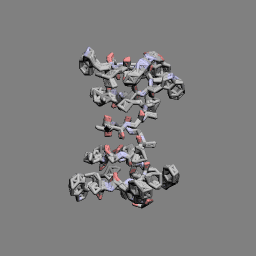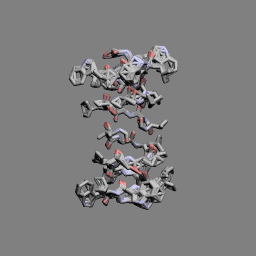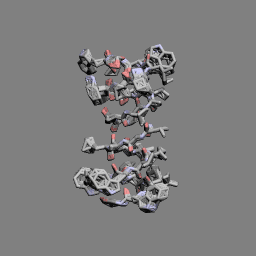 Gramicidin A head-to-head dimer | |
| Identifiers | |
|---|---|
| Symbol | N/A |
| TCDB | 1.D.1 |
| OPM superfamily | 65 |
| OPM protein | 1grm |
 Structure of gramicidin A, B, and C (click to enlarge) | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.014.355 |
| Chemical and physical data | |
| Formula | C99H140N20O17 |
| Molar mass | 1882.332 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 229 to 230 °C (444 to 446 °F) [1] |
| Solubility in water | 0.006 mg/L[1] |
| |
| |
| | |
Gramicidin, also called gramicidin D, is a mix of ionophoric antibiotics, gramicidin A, B and C, which make up about 80%, 5%, and 15% of the mix, respectively. Each has 2 isoforms, so the mix has 6 different types of gramicidin molecules. They can be extracted from Brevibacillus brevis soil bacteria. Gramicidins are linear peptides with 15 amino acids.[2] This is in contrast to unrelated gramicidin S, which is a cyclic peptide.
Medical uses
Gramicidins work as antibiotics against gram-positive bacteria like Bacillus subtilis and Staphylococcus aureus, but not well against gram-negative ones like E. coli.[3]
Gramicidins are used in medicinal lozenges for sore throat and in topical medicines to treat infected wounds. Gramicidins are often mixed with other antibiotics like tyrocidine and antiseptics.[4] Gramicidins are also used in eye drops for bacterial eye infections. In drops, they are often mixed with other antibiotics like polymyxin B or neomycin. Multiple antibiotics increase efficiency against various strains of bacteria.[5] Such eye-drops are also used to treat eye infections of animals, like horses.[6]
History
In 1939, René Dubos isolated the substance tyrothricin.[7][8] Later this was shown to be a mix of gramicidin and tyrocidine. These were the first antibiotics to be manufactured commercially.[8] Letter "D" in gramicidin D is short for "Dubos",[9] and was invented to differentiate the mix from gramicidin S.[10]
In 1964, the sequence of gramicidin A was determined by Reinhard Sarges and Bernhad Witkop.[11][12]
In 1971, the dimeric head-to-head structure of gramicidins was proposed by D. W. Urry.[13]
In 1993, the structure of the gramicidin head-to-head dimer in micelles and lipid bilayers was determined by solution and solid-state NMR.[14]
Structure and chemistry
Gramicidins A, B and C are nonribosomal peptides, thus they have no genes. They consist of 15 L- and D-amino acids. Their amino acid sequence is:[2]
- formyl-L-X-Gly-L-Ala-D-Leu-L-Ala-D-Val-L-Val-D-Val-L-Trp-D-Leu-L-Y-D-Leu-L-Trp-D-Leu-L-Trp-ethanolamine
Y is L-tryptophan in gramicidin A, L-phenylalanine in B and L-tyrosine in C. X determines isoform. X is L-valine or L-isoleucine – in natural gramicidin mixes of A, B and C, about 5% of the total gramicidins are isoleucine isoforms.[2]
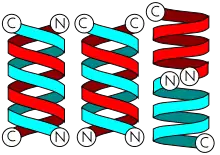
Gramicidins form helices. The alternating pattern of D- and L-amino acids is important for the formation of these structures. Helices occur most often as head-to-head dimers. 2 gramicidins can also form antiparallel or parallel double helices, especially in organic solvents. Dimers are long enough to span cellular lipid bilayers and thus function as ion channel -type of ionophores.[12]
Gramicidin mixture is a crystalline solid. Its solubility in water is minimal, 6 mg/L, and it may form colloidal suspensions. It is soluble in small alcohols, acetic acid, pyridine, poorly soluble in acetone and dioxane, and practically insoluble in diethylether and hydrocarbons.[1]
Pharmacological effect
Gramicidins are ionophores. Their dimers form ion channel-like pores in cell membranes and cellular organelles of bacteria and animal cells.[15] Inorganic monovalent ions, such as potassium (K+) and sodium (Na+), can travel through these pores freely via diffusion. This destroys vital ion concentration differences, i.e. ion gradients, between membranes thereby killing the cell via various effects. For example, ion leak in mitochondria halts mitochondrial ATP production in cells with mitochondria.[16]
Gramicidins can be used as topical antibiotic medications in low doses, even though they are potentially lethal for human cells. Bacteria die at lower gramicidin concentrations than human cells.[3] Gramicidins are not used internally, as their significant intake may cause hemolysis and be toxic to the liver, kidney, meninges and olfactory system among other effects.[16]
References
- 1 2 3 Budavari S (1996). The Merck index: an encyclopedia of chemicals, drugs, and biologicals (12th ed.). Merck. p. 712. ISBN 0911910123. OCLC 34552962.
- 1 2 3 Kessler N, Schuhmann H, Morneweg S, Linne U, Marahiel MA (February 2004). "The linear pentadecapeptide gramicidin is assembled by four multimodular nonribosomal peptide synthetases that comprise 16 modules with 56 catalytic domains". The Journal of Biological Chemistry. 279 (9): 7413–9. doi:10.1074/jbc.M309658200. PMID 14670971.
- 1 2 Wang F, Qin L, Pace CJ, Wong P, Malonis R, Gao J (January 2012). "Solubilized gramicidin A as potential systemic antibiotics". ChemBioChem. 13 (1): 51–5. doi:10.1002/cbic.201100671. PMID 22113881. S2CID 4906040.
- ↑ Palm J, Fuchs K, Stammer H, Schumacher-Stimpfl A, Milde J (December 2018). "Efficacy and safety of a triple active sore throat lozenge in the treatment of patients with acute pharyngitis: Results of a multi-centre, randomised, placebo-controlled, double-blind, parallel-group trial (DoriPha)". International Journal of Clinical Practice. 72 (12): e13272. doi:10.1111/ijcp.13272. PMC 6282512. PMID 30329199.
- ↑ Bosscha MI, van Dissel JT, Kuijper EJ, Swart W, Jager MJ (January 2004). "The efficacy and safety of topical polymyxin B, neomycin and gramicidin for treatment of presumed bacterial corneal ulceration". The British Journal of Ophthalmology. 88 (1): 25–8. doi:10.1136/bjo.88.1.25. PMC 1771930. PMID 14693766.
- ↑ Gilger BC, Allbaugh RA (2011). Equine ophthalmology (2nd ed.). Elsevier Saunders. pp. 111, 190. ISBN 9781437708462.
- ↑ Dubos RJ (June 1939). "Studies on a bactericidal agent extracted from a soil bacillus: I. Preparation of the agent. Its activity in vitro". The Journal of Experimental Medicine. 70 (1): 1–10. doi:10.1084/jem.70.1.1. PMC 2133784. PMID 19870884.
- 1 2 Van Epps HL (February 2006). "René Dubos: unearthing antibiotics". The Journal of Experimental Medicine. 203 (2): 259. doi:10.1084/jem.2032fta. PMC 2118194. PMID 16528813.
- ↑ Lum K (2017). "Exchange of gramicidin between lipid bilayers: implications for the mechanism of channel formation". Biophysical Journal. 113 (8): 1757–1767. Bibcode:2017BpJ...113.1757L. doi:10.1016/j.bpj.2017.08.049. ISSN 0006-3495. PMC 5647621. PMID 29045870.
- ↑ "Gramicidin from Bacillus brevis product No. G 5002" (PDF). sigmaaldrich.com. 1997-09-06. Retrieved 2019-10-03.
- ↑ Sarges R, Bernhard W (1964). "gramicidin A. IV. Primary sequence of valine and isoleucine gramicidin A". Journal of the American Chemical Society. 86 (9): 1862–1863. doi:10.1021/ja01063a049. ISSN 0002-7863.
- 1 2 3 Meikle TG, Conn CE, Separovic F, Drummond CJ (2016). "Exploring the structural relationship between encapsulated antimicrobial peptides and the bilayer membrane mimetic lipidic cubic phase: studies with gramicidin A′". RSC Advances. 6 (73): 68685–68694. Bibcode:2016RSCAd...668685M. doi:10.1039/C6RA13658C.
- ↑ Urry DW (March 1971). "The gramicidin A transmembrane channel: a proposed pi(L,D) helix". Proceedings of the National Academy of Sciences of the United States of America. 68 (3): 672–6. Bibcode:1971PNAS...68..672U. doi:10.1073/pnas.68.3.672. PMC 389014. PMID 5276779.
- ↑ Ketchem RR, Hu W, Cross TA (September 1993). "High-resolution conformation of gramicidin A in a lipid bilayer by solid-state NMR". Science. 261 (5127): 1457–60. Bibcode:1993Sci...261.1457K. doi:10.1126/science.7690158. PMID 7690158.
- ↑ Sorochkina AI, Plotnikov EY, Rokitskaya TI, Kovalchuk SI, Kotova EA, Sychev SV, et al. (2012). "N-terminally glutamate-substituted analogue of gramicidin A as protonophore and selective mitochondrial uncoupler". PLOS ONE. 7 (7): e41919. Bibcode:2012PLoSO...741919S. doi:10.1371/journal.pone.0041919. PMC 3404012. PMID 22911866.
- 1 2 David JM, Rajasekaran AK (2015). "Gramicidin A: A New Mission for an Old Antibiotic". Journal of Kidney Cancer and VHL. 2 (1): 15–24. doi:10.15586/jkcvhl.2015.21. PMC 5345515. PMID 28326255.