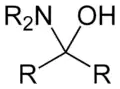
In organic chemistry, a hemiaminal (also carbinolamine) is a functional group or type of chemical compound that has a hydroxyl group and an amine attached to the same carbon atom: −C(OH)(NR2)−. R can be hydrogen or an alkyl group. Hemiaminals are intermediates in imine formation from an amine and a carbonyl by alkylimino-de-oxo-bisubstitution.[1] Hemiaminals can be viewed as a blend of aminals and geminal diol. They are a special case of amino alcohols.
Classification according to amine precursor
Hemiaminals form from the reaction of an amine and a ketone or aldehyde. The hemiaminal is sometimes isolable, but often they spontaneously dehydrate to give imines.[2]
Addition of ammonia
The adducts formed by the addition of ammonia to aldehydes have long been studied.[3] Compounds containing both a primary amino group and a hydroxyl group bonded to the same carbon atom are rarely stable, as they tend to dehydrate to form imines which polymerise to hexamethylenetetramine. A rare stable example is the adduct of ammonia and hexafluoroacetone, (CF3)2C(OH)NH2.[4]
The C-substituted derivatives are obtained by reaction of aldehydes and ammonia:[5]
Addition of primary amines
N-substituted derivatives are somewhat stable. They are invoked but rarely observed as intermediates in the Mannich reaction. These N,N',N''-trisubstituted hexahydro-1,3,5-triazines arise from the condensation of the amine and formaldehyde as illustrated by the route to 1,3,5-trimethyl-1,3,5-triazacyclohexane:
Although adducts generated from primary amines or ammonia are usually unstable, the hemiaminals have been trapped in a cavity.[6]
Addition of secondary amines: carbinolamines (hemiaminals) and bisaminomethanes
One of the simplest reactions entails condensation of formaldehyde and dimethylamine. This reaction produces first the carbinolamine (a hemiaminal) and bis(dimethylamino)methane (Me = CH3):[7][8]
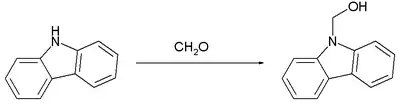
The reaction of formaldehyde with carbazole, which is weakly basic, proceed similarly:[9]
Again, this carbinol converts readily to the methylene-linked bis(carbazole).
Hemiaminal ethers
Hemiaminal ethers have the following structure: R‴-C(NR'2)(OR")-R⁗. The glycosylamines are examples of cyclic hemiaminal ethers.
- Hemiaminals and ethers
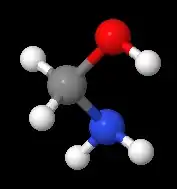
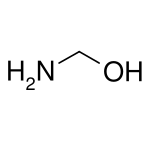 methanolamine, an intermediate in the reaction of ammonia with formaldehyde
methanolamine, an intermediate in the reaction of ammonia with formaldehyde2.png.webp) Bis(hydroxymethyl)urea is a commercially useful hemiaminal
Bis(hydroxymethyl)urea is a commercially useful hemiaminal An unusual example of an isolable, acyclic hemiaminal: the adduct of ammonia and hexafluoroacetone
An unusual example of an isolable, acyclic hemiaminal: the adduct of ammonia and hexafluoroacetone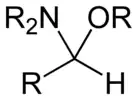 Hemiaminal ether derived from an aldehyde
Hemiaminal ether derived from an aldehyde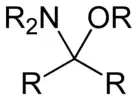 Hemiaminal ether derived from a ketone
Hemiaminal ether derived from a ketone2.svg.png.webp)
Use in total synthesis
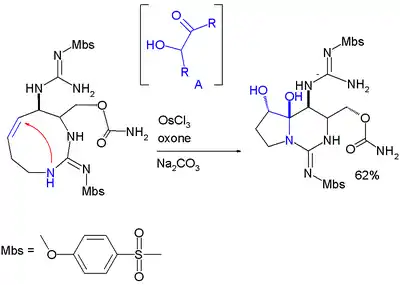
Hemiaminal formation is a key step in an asymmetric total synthesis of saxitoxin:[10]
In this reaction step the alkene group is first oxidized to an intermediate acyloin by action of osmium(III) chloride, oxone (sacrificial catalyst) and sodium carbonate (base).
See also
References
- ↑ Urbansky, Edward T. (2000). "Carbinolamines and Geminal Diols in Aqueous Environmental Organic Chemistry". Journal of Chemical Education. 77 (12): 1644. Bibcode:2000JChEd..77.1644U. doi:10.1021/ed077p1644.
- ↑ Gabbutt, Christopher D.; Hepworth, John D. (1995). "Functions Incorporating a Chalcogen and a Group 15 Element". Comprehensive Organic Functional Group Transformations. pp. 293–349. doi:10.1016/B0-08-044705-8/00206-5. ISBN 9780080447056.
- ↑ Justus Liebig "Ueber die Producte der Oxydation des Alkohols" Annalen der Pharmacie 1835, Volume 14, pp 133–167. doi:10.1002/jlac.18350140202
- ↑ W. J. Middleton, H. D. Carlson (1970). "Hexafluoroacetone imine". Org. Syntheses. 50: 81–3. doi:10.15227/orgsyn.050.0081..
- ↑ Nielsen, Arnold T.; Atkins, Ronald L.; Moore, Donald W.; Scott, Robert; Mallory, Daniel; LaBerge, Jeanne M. (1973). "Structure and Chemistry of the Aldehyde Ammonias. 1-Amino-1-Alkanols, 2,4,6-Trialkyl-1,3,5-Hexahydrotriazines, and N,N-Dialkylidene-1,1-Diaminoalkanes". J. Org. Chem. 38 (19): 3288–3295. doi:10.1021/jo00959a010.
- ↑ Iwasawa, T.; Hooley, R. J.; Rebek, J. (2007). "Stabilization of Labile Carbonyl Addition Intermediates by a Synthetic Receptor". Science. 317 (5837): 493–496. Bibcode:2007Sci...317..493I. doi:10.1126/science.1143272. PMID 17656719. S2CID 37292853.
- ↑ Hellmann Heinrich, Günter Opitz (1960). Aminoalkylierung. Weinheim.
{{cite book}}: CS1 maint: location missing publisher (link) - ↑ Rogers, F. E.; Rapiejko, R. J. (1974). "Thermochemistry of Carbonyl Addition Reactions. II. Enthalpy of Addition of Dimethylamine to Formaldehyde". The Journal of Physical Chemistry. 78 (6): 599–603. doi:10.1021/j100599a008.
- ↑ Carbazol-9-yl-methanol Milata Viktora, Kada Rudolfa, Lokaj J¨¢nb Molbank 2004, M354 open access publication Archived 2018-09-26 at the Wayback Machine
- ↑ Fleming, James J.; McReynolds, Matthew D.; Du Bois, J. (2007). "(+)-Saxitoxin: A First and Second Generation Stereoselective Synthesis". Journal of the American Chemical Society. 129 (32): 9964–9975. doi:10.1021/ja071501o. PMID 17658800.