 | |
| Clinical data | |
|---|---|
| Trade names | Jublia, Clenafin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a614050 |
| License data |
|
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.245.862 |
| Chemical and physical data | |
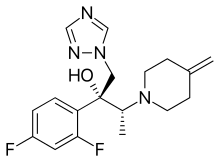
| Formula | C18H22F2N4O |
| Molar mass | 348.398 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Efinaconazole, sold under the brand name Jublia among others, is a triazole antifungal medication. It is approved for use in the United States, Canada, and Japan as a 10% topical solution for the treatment of onychomycosis (fungal infection of the nail).[3][4] Efinaconazole acts as a 14α-demethylase inhibitor.[5][2]
It is available as a generic medication.[6][7][8][9]
Medical uses
Efinaconazole is an azole antifungal indicated in the US for the topical treatment of onychomycosis of the toenails due to Trichophyton rubrum and Trichophyton mentagrophytes.[2]
Efficacy
In two clinical trials 17.8% (trial 1) and 15.2% (trial 2) of participants using efinaconazole were completely cured (0% clinical involvement of the target toenail, plus negative KOH test and negative culture), compared with 3.3% (trial 1) and 5.5% (trial 2) of participants using a placebo.[2] The "complete cure or almost complete cure" rate (≤5% affected target toenail area involved, and negative KOH and culture) for efinaconazole was 26.4% (trial 1) and 23.4% (trial 2) (compared with 7.0% (trial 1) and 7.5% (trial 2)).[2]
History
In 2014, the U.S. Food and Drug Administration (FDA) approved the New Drug Application (NDA).[10][11] According to Valeant Pharmaceuticals International Inc CEO J. Michael Pearson they acquired Jublia through their purchase of Dow Pharmaceutical Sciences in 2008.[11]
In 2020, the FDA approved a supplemental New Drug Application for efinaconazole topical solution, 10%, which extended the age range included in the product's label to children six years of age and older; it was first approved in 2014, in people aged 18 years of age and older.[12]
Society and culture
Economics
In 2015, the cost of treatment with efinaconazole in the United States was said to be US$2,307 per nail.[13]
In 2019, a study by the Canadian Agency for Drugs and Technologies in Health found the cost for a 48-week course to be $178 for a big toe, and $89 for an other toe.[14]
References
- ↑ "Archived copy" (PDF). Archived (PDF) from the original on 10 June 2022. Retrieved 10 June 2022.
{{cite web}}: CS1 maint: archived copy as title (link) - 1 2 3 4 5 "Jublia- efinaconazole solution". DailyMed. 30 September 2016. Archived from the original on 30 November 2020. Retrieved 27 April 2020.
- ↑ Patel T, Dhillon S (November 2013). "Efinaconazole: first global approval". Drugs. 73 (17): 1977–1983. doi:10.1007/s40265-013-0152-x. PMID 24249649. S2CID 40015633.
- ↑ Tschen EH, Bucko AD, Oizumi N, Kawabata H, Olin JT, Pillai R (February 2013). "Efinaconazole solution in the treatment of toenail onychomycosis: a phase 2, multicenter, randomized, double-blind study". J Drugs Dermatol. 12 (2): 186–192. PMID 23377392.
- ↑ Tatsumi Y, Nagashima M, Shibanushi T, et al. (May 2013). "Mechanism of action of efinaconazole, a novel triazole antifungal agent". Antimicrob Agents Chemother. 57 (5): 2405–2509. doi:10.1128/aac.02063-12. PMC 3632939. PMID 23459486.
- ↑ "Efinaconazole: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 21 March 2021. Retrieved 14 February 2021.
- ↑ "Efinaconazole: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 21 March 2021. Retrieved 14 February 2021.
- ↑ "First Generic Drug Approvals". U.S. Food and Drug Administration (FDA). Archived from the original on 26 January 2021. Retrieved 13 February 2021.
- ↑ "Competitive Generic Therapy Approvals". U.S. Food and Drug Administration (FDA). 3 March 2023. Retrieved 6 March 2023.
- ↑ "Drug Approval Package: Jublia topical solution (efinaconazole) NDA #203567". U.S. Food and Drug Administration (FDA). 24 December 1999. Archived from the original on 29 May 2020. Retrieved 27 April 2020.
- 1 2 "Valeant Pharmaceuticals Announces FDA Approval Of Jublia for the Treatment of Onychomycosis". Valeant Pharmaceuticals (Press release). 9 June 2014. Archived from the original on 8 November 2015. Retrieved 1 November 2015.
- ↑ "FDA Approves Ortho Dermatologics' Labeling For Jublia (efinaconazole) Topical Solution, 10%, In Patients As Young As Six Years Old". Bausch Health (Press release). Archived from the original on 10 June 2022. Retrieved 10 June 2022.
- ↑ Mikailov A, Cohen J, Joyce C, Mostaghimi A (2015). "Cost-effectiveness of Confirmatory Testing Before Treatment of Onychomycosis". JAMA Dermatology. 152 (3): 1–6. doi:10.1001/jamadermatol.2015.4190. PMID 26716567.
- ↑ "Table 5, CDR Cost Comparison Table for Onychomycosis". www.ncbi.nlm.nih.gov. 8 June 2019. Archived from the original on 10 June 2022. Retrieved 10 June 2022.
External links
- "Efinaconazole". Drug Information Portal. U.S. National Library of Medicine.