 | |
| Names | |
|---|---|
| Other names
Diludine, 1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic acid diethyl ester | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.013.237 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H19NO4 | |
| Molar mass | 253.298 g·mol−1 |
| Appearance | white or colorless solid |
| Melting point | 182–183 °C (360–361 °F; 455–456 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Hantzsch ester refers to an organic compound with the formula HN(MeC=C(CO2Et))2CH2 where Me = methyl (CH3) and Et = ethyl (C2H5). It is a light yellow solid. The compound is an example of a 1,4-dihydropyridine. It is named after Arthur Rudolf Hantzsch who described its synthesis in 1881. The compound is a hydride donor, e.g., for reduction of imines to amines. It is a synthetic analogue of NADH, a naturally occurring dihydropyridine.[1]
Preparation
Hantzsch ester can be made with a Hantzsch pyridine synthesis where formaldehyde, two equivalents of ethyl acetoacetate and ammonium acetate are combined to afford the product in high yield.[2]
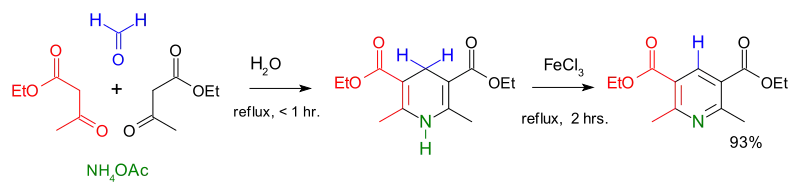
Hantzsch reaction with ammonium acetate, ethyl acetoacetate, formaldehyde and ferric chloride
Structure
As confirmed by X-ray crystallography, Hantzsch ester has a planar C5N core.[3]
Further reading
See also
References
- ↑ Bechara, William S.; Charette, André B.; Na, Risong; Wang, Wenliang; Zheng, Chao (2020). "Diethyl 1,4‐Dihydro‐2,6‐dimethyl‐3,5‐Pyridinedicarboxylate". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn01318. ISBN 978-0471936237.
- ↑ Cheung, Lawrence L. W.; Styler, Sarah A.; Dicks, Andrew P. (2010). "Rapid and Convenient Synthesis of the 1,4-Dihydropyridine Privileged Structure". Journal of Chemical Education. 87 (6): 628–630. Bibcode:2010JChEd..87..628C. doi:10.1021/ed100171g.
- ↑ Stockinger, Skrollan; Troendlin, Johannes; Rominger, Frank; Trapp, Oliver (2015). "On-Column Reaction Set-Up for High-Throughput Screenings and Mechanistic Investigations". Advanced Synthesis & Catalysis. 357 (16–17): 3513–3520. doi:10.1002/adsc.201500311.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.