Grayanotoxins are a group of closely related neurotoxins named after Leucothoe grayana, a plant native to Japan originally named for 19th century American botanist Asa Gray.[1] Grayanotoxin I (grayanotoxane-3,5,6,10,14,16-hexol 14-acetate) is also known as andromedotoxin, acetylandromedol, rhodotoxin and asebotoxin.[2] Grayanotoxins are produced by Rhododendron species and other plants in the family Ericaceae. Honey made from the nectar and so containing pollen of these plants also contains grayanotoxins and is commonly referred to as mad honey.[3] Consumption of the plant or any of its secondary products, including mad honey, can cause a rare poisonous reaction called grayanotoxin poisoning, mad honey disease, honey intoxication, or rhododendron poisoning.[3][4] It is most frequently produced and consumed in regions of Nepal and Turkey as a recreational drug and traditional medicine.[5]
Origin

Grayanotoxins are produced by plants in the family Ericaceae, specifically members of the genera Rhododendron, Pieris, Agarista and Kalmia.[3] The genus Rhododendron alone encompasses over 750 species that grow around the world in parts of Europe, North America, Japan, Nepal and Turkey. They can grow at a variety of altitudes ranging from sea level to more than three kilometers above. While many of these species contain grayanotoxins, only a few contain significant levels. Species with high concentrations of grayanotoxins such as R. ponticum, R. flavum and R. luteum are most commonly found in Nepal and regions of Turkey bordering the Black Sea.[5]
.jpg.webp)
Nearly all parts of grayanotoxin-producing rhododendrons contain the molecule, including the stem, leaves, flower, pollen and nectar. Grayanotoxins can also be found in secondary plant products such as honey, labrador tea, cigarettes and herbal medicines.[3]
Chemical structure
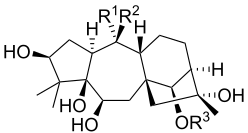
| Grayanotoxin | R1 | R2 | R3 |
| Grayanotoxin I | OH | CH3 | Ac |
| Grayanotoxin II | CH2 | H | |
| Grayanotoxin III | OH | CH3 | H |
| Grayanotoxin IV | CH2 | Ac | |
Grayanotoxins are low molecular weight hydrophobic compounds.[6] They are structurally characterized as polyhydroxylated cyclic diterpenes. The base structure is a 5/7/6/5 ring system that does not contain nitrogen.[3] More than 25 grayanotoxin isoforms have been identified from Rhododendron species[5], but grayanotoxin I and III are thought to be the principal toxic isoforms. Different Rhododendron species contain multiple different grayanotoxin isoforms, contributing to differences in plant toxicity.[3]
Mechanism of action
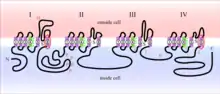
The toxicity of grayanotoxin is derived from its ability to interfere with voltage-gated sodium channels located in the cell membrane of neurons. The Nav1.x channels consist of four homologous domains (I-IV), each containing six transmembrane alpha-helical segments (S1-S6). Grayanotoxin has a binding affinity (IC50) of approximately 10 μM and binds the group II receptor site located on segment 6 of domains I and IV (IS6 and IVS6).[3] Other toxins that bind to this region include the alkaloids veratridine, batrachotoxin and aconitine.[6]
Experiments utilizing squid axonal membranes indicate that sodium channel binding likely occurs on the internal face of the neuron.[7] Additionally, grayanotoxin only binds to the activated conformation of sodium channels. Normally, voltage gated sodium channels are activated (opened) only when the cell membrane potential reaches a specific threshold voltage. This activated conformation allows for an influx of sodium ions resulting in cell depolarization, followed by the firing of an action potential. At the peak of the action potential, voltage-gated sodium channels are quickly inactivated and are only reset once the cell has repolarized to resting potential. When grayanotoxin is present, binding induces further conformational changes that prevent sodium channel inactivation and lead to a prolonged depolarization. Owing to its transient ability to activate channels and increase membrane permeability to sodium ions, grayanotoxin is classified as a reversible Nav1.x agonist.[6]
Biological effects
Prolonged sodium channel activation and cell depolarization leads to overstimulation of the central nervous system. Physical symptoms from grayanotoxin poisoning appear after a dose-dependent latent period of several minutes to approximately three hours. The most common clinical symptoms include various cardiovascular effects, nausea and vomiting, and a change in consciousness. The cardiovascular effects may include hypotension (low blood pressure) and various cardiac rhythm disorders such as sinus bradycardia (slow regular heart rhythm), bradyarrhythmia (slow irregular heart rhythm) and partial or complete atrioventricular block.[3][8]
Other early-onset symptoms may include diplopia and blurred vision, dizziness, hypersalivation, perspiration, weakness and paresthesia in the extremities and around the mouth. In higher doses, symptoms can include loss of coordination, severe and progressive muscular weakness, electrocardiographic changes of bundle branch block and/or ST-segment elevations as seen in ischemic myocardial threat, and nodal rhythm or Wolff-Parkinson-White syndrome.[9]
The primary mediator of this grayanotoxin pathophysiology is the paired vagus nerve (tenth cranial nerve).[3] The vagus nerve is a major component of the parasympathetic nervous system (a branch of the autonomic nervous system) and innervates various organs including the lungs, stomach, kidney and heart. In one study, experimental administration of grayanotoxin to bilaterally vagotomized rats failed to induce bradycardia, a common symptom of grayanotoxin poisoning, supporting the role of vagal stimulation.[10] Vagal stimulation of the myocardium, specifically, is mediated by M2-subtype muscarinic acetylcholine receptors (mAChR).[11] In severe cases of grayanotoxin poisoning, atropine (a non-specific "mAChR antagonist" or Muscarinic antagonist) can be used to treat bradycardia and other heart rhythm malfunctions. In addition to correcting rhythm disorders, administration of fluids and vasopressors can also help treat hypotension and mitigate other symptoms.[12]
Patients exposed to low doses of grayanotoxin typically recover within a few hours. In more severe cases, symptoms may persist for 24 hours or longer and may require medical treatment (as described above). Despite the risk from cardiac problems, grayanotoxin poisoning is rarely fatal in humans.[12]
Animal poisoning
In contrast to humans, grayanotoxin poisoning can be lethal for other animals.[3] Nectar containing grayanotoxin can kill honeybees, though some seem to have resistance to it and can produce honey from the nectar (see below). According to a team of researchers from the UK and Ireland, worker bumblebees are not harmed and may be preferable as pollinators because they transfer more pollen. Consequently, it may be advantageous for plants to produce grayanotoxin in order to be pollinated by bumblebees.[13]
Mad honey intoxication
Bees that collect pollen and nectar from grayanotoxin-containing plants often produce honey that also contains grayanotoxins.[3][8] This so-called "mad honey" is the most common cause of grayanotoxin poisoning in humans. Small-scale producers of mad honey typically harvest honey from a small area or single hive in order to produce a final product containing a significant concentration of grayanotoxin. In contrast, large-scale honey production often mixes honey gathered from different locations, diluting the concentration of any contaminated honey.[8]
Mad honey is deliberately produced in some regions of the world, most notably Nepal and the Black Sea region of Turkey. In Nepal, this type of honey is used by the Gurung people for both its hallucinogenic properties and supposed medicinal benefits.[14] In Turkey, mad honey known as deli bal is also used as a recreational drug and traditional medicine. It is most commonly made from the nectar of Rhododendron luteum and Rhododendron ponticum in the Caucasus region.[15] In the eighteenth century, this honey was exported to Europe to add to alcoholic drinks to give them extra potency. In modern times, it is consumed locally and exported to North America, Europe and Asia.[8][16][17]
In addition to various Rhododendron species, mad honey can also be made from several other grayanotoxin-containing plants. Honey produced from the nectar of Andromeda polifolia contains high enough levels of grayanotoxin to cause full body paralysis and potentially fatal breathing difficulties due to diaphragm paralysis.[8][18] Honey obtained from spoonwood and allied species such as sheep-laurel can also cause illness.[8] The honey from Lestrimelitta limao also produces this paralyzing effect seen in the honey of A. polifolia and is also toxic to humans.[19]
Although mad honey is used in traditional medicine in Turkey,[3] the majority of grayanotoxin poisoning cases occur in middle-aged males who use the honey for perceived sexual enhancement.[20]
Historical use
The intoxicating effects of mad honey have been known for thousands of years. There have been many famous episodes of human inebriation caused by its consumption. Xenophon, Aristotle, Strabo, Pliny the Elder[16][21] and Columella all document the results of eating this "maddening" honey, believed to be from the pollen and nectar of Rhododendron luteum and Rhododendron ponticum.[22] According to Xenophon's Anabasis, an invading Greek army was accidentally poisoned by harvesting and eating the local Asia Minor honey, but they all made a quick recovery without any fatalities.[23] Having heard of this incident, and realizing that foreign invaders would be ignorant of the dangers of the local honey, King Mithridates later used the honey as a deliberate poison when Pompey's army attacked the Heptakometes in Asia Minor in 69 BC.[24] The Roman soldiers became delirious and nauseated after being tricked into eating the toxic honey, at which point Mithridates's army attacked.[25][26][27]
Popular culture
In A Haunting in Venice, a 2023 murder mystery film based on Agatha Christie's 1969 novel Hallowe'en Party, the honey is used to poison the first victim. The protagonist Hercule Poirot is also drugged with it, causing him to hallucinate and ponder the existence of ghosts. The drug is also featured in the episode "New Jazz" of the television series Atlanta. In Angel Dust (film), a 1994 thriller horror film directed by Japanese filmmaker Gakuryū Ishii the murders are all done by injected rhodotoxin, causing near instant death, which within the film is discovered in forensic bloodwork, and said to have been available at any plant store. The song "Rhododendron Honey" by Leslie Fish is about a mountain community that uses mad honey to poison outsiders. The "mad honey disease" is equally a key element into the movie "Sherlock Holmes" (2009) by Guy Ritchie.
See also
References
- ↑ Senning A (2007). Elsevier's Dictionary of Chemoetymology. Amsterdam: Elsevier. p. 170. ISBN 978-0-444-52239-9.
- ↑ The Merck Index (10th ed.). Rahway, NJ: Merck. 1983. pp. 652–653. ISBN 9780911910278.
- 1 2 3 4 5 6 7 8 9 10 11 12 Jansen SA, Kleerekooper I, Hofman ZL, Kappen IF, Stary-Weinzinger A, van der Heyden MA (September 2012). "Grayanotoxin poisoning: 'mad honey disease' and beyond". Cardiovascular Toxicology. 12 (3): 208–15. doi:10.1007/s12012-012-9162-2. PMC 3404272. PMID 22528814.
- ↑ Demircan A, Keleş A, Bildik F, Aygencel G, Doğan NO, Gómez HF (December 2009). "Mad honey sex: therapeutic misadventures from an ancient biological weapon". Annals of Emergency Medicine. 54 (6): 824–9. doi:10.1016/j.annemergmed.2009.06.010. PMID 19683834.
- 1 2 3 Sahin H (18 April 2015). "Grayanotoxin-III Detection and Antioxidant Activity of Mad Honey". International Journal of Food Properties. 18 (12): 2665–2674. doi:10.1080/10942912.2014.999866. S2CID 97859238.
- 1 2 3 Sperelakis N (2011). Cell Physiology Source Book: Essentials of Membrane Biophysics. Elsevier Science & Technology. pp. 510–513. ISBN 9780123877383.
- ↑ Seyama I, Yamada K, Kato R, Masutani T, Hamada M (February 1988). "Grayanotoxin opens Na channels from inside the squid axonal membrane". Biophysical Journal. 53 (2): 271–4. Bibcode:1988BpJ....53..271S. doi:10.1016/s0006-3495(88)83088-1. PMC 1330147. PMID 2449919.
- 1 2 3 4 5 6 "Grayanotoxins". Foodborne Pathogenic Microorganisms and Natural Toxins Handbook. US FDA. 2012. Retrieved August 7, 2015.
- ↑ Sayin MR, Karabag T, Dogan SM, Akpinar I, Aydin M (April 2012). "Transient ST segment elevation and left bundle branch block caused by mad-honey poisoning". Wiener Klinische Wochenschrift. 124 (7–8): 278–81. doi:10.1007/s00508-012-0152-y. PMID 22527815. S2CID 21598407.
- ↑ Onat F, Yegen BC, Lawrence R, Oktay A, Oktay S (June 1991). "Site of action of grayanotoxins in mad honey in rats". Journal of Applied Toxicology. 11 (3): 199–201. doi:10.1002/jat.2550110308. PMID 1918794. S2CID 30333456.
- ↑ Onat FY, Yegen BC, Lawrence R, Oktay A, Oktay S (1991). "Mad honey poisoning in man and rat". Reviews on Environmental Health. 9 (1): 3–9. doi:10.1515/reveh.1991.9.1.3. hdl:11424/218274. PMID 1957047. S2CID 12261007.
- 1 2 "Bad Bug Book: Handbook of Foodborne Pathogenic Microorganisms and Natural Toxins" (PDF). FDA. Retrieved 3 May 2018.
- ↑ Stephanie Pain (Apr 25, 2015). "Bitter sweet nectar: Why some flowers poison bees". New Scientist.
- ↑ Treza R (2011). "Hallucinogen honey hunters". topdocumentaryfilms.com. Retrieved 20 October 2015.
- ↑ Waters J (1 October 2014). "The buzz about 'mad honey', hot honey and mead". The Guardian.
- 1 2 Mayor A. "Mad Honey!". Archaeology. 46 (6): 32–40.
- ↑ Williams C (2010). Medicinal Plants in Australia Volume 1: Bush Pharmacy. Rosenberg Publishing. p. 223. ISBN 978-1877058790.
- ↑ Lensky Y (1997). Bee Products: Properties, Applications, and Apitherapy. Springer. ISBN 0-306-45502-1.
- ↑ Wittmann D, Radtke R, Zeil J, Lübke G, Francke W (February 1990). "Robber bees (Lestrimelitta limao) and their host chemical and visual cues in nest defense byTrigona (Tetragonisca) angustula (Apidae: Meliponinae)". Journal of Chemical Ecology. 16 (2): 631–41. doi:10.1007/bf01021793. PMID 24263518. S2CID 34424143.
- ↑ Eroğlu SE, Urgan O, Onur OE, Denizbaşı A, Akoğlu H (September 2013). "Grayanotoxin (mad honey) - ongoing consumption after poisoning". Balkan Medical Journal. 30 (3): 293–5. doi:10.5152/balkanmedj.2013.8100. PMC 4115918. PMID 25207122.
- ↑ Pliny the Elder. "21.45—Maddening honey". Natural History.
- ↑ Kelhoffer JA (2005). "John the Baptist's "Wild Honey" and "Honey" in Antiquity". Greek, Roman, and Byzantine Studies. 45: 59–73.
- ↑ Xenophon. "4.8.19–21". In Brownson CL (ed.). Anabasis. Department of the Classics, Tufts University.
{{cite book}}:|work=ignored (help) - ↑ Lane RW, Borzelleca JF (2007). "Harming and Helping Through Time: The History of Toxicology". In Hayes AW (ed.). Principles and methods of toxicology (5th ed.). Boca Raton: Taylor & Francis. ISBN 978-0-8493-3778-9.
- ↑ Strabo. "12.3.18". Geography.
- ↑ Georghiou GP (1980). "Ancient Beekeeping". In Root, A.I. (ed.). The ABC and XYZ of Bee Culture. Medina, Ohio: A.I. Root Company. pp. 17–21.
- ↑ Ambrose JT (1972). Bees and Warfare: Gleanings in Bee Culture. pp. 343–6.