| methylmalonyl CoA epimerase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC no. | 5.1.99.1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| methylmalonyl CoA epimerase | |||||||
|---|---|---|---|---|---|---|---|
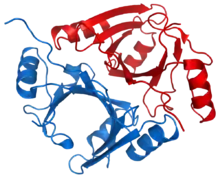
 Methylmalonyl-CoA epimerase homodimer (mitochondrial), Human | |||||||
| Identifiers | |||||||
| Symbol | MCEE | ||||||
| NCBI gene | 84693 | ||||||
| HGNC | 16732 | ||||||
| OMIM | 608419 | ||||||
| RefSeq | NM_028626 | ||||||
| UniProt | Q96PE7 | ||||||
| Other data | |||||||
| EC number | 5.1.99.1 | ||||||
| Locus | Chr. 2 p13.3 | ||||||
| |||||||
Methylmalonyl CoA epimerase (EC 5.1.99.1, methylmalonyl-CoA racemase, methylmalonyl coenzyme A racemase, DL-methylmalonyl-CoA racemase, 2-methyl-3-oxopropanoyl-CoA 2-epimerase [incorrect]) is an enzyme involved in fatty acid catabolism that is encoded in human by the "MCEE" gene located on chromosome 2. It is routinely and incorrectly labeled as "methylmalonyl-CoA racemase". It is not a racemase because the CoA moiety has 5 other stereocenters.
Structure
The "MCEE" gene is located in the 2p13 region and contains 4 exons, and encodes for a protein that is approximately 18 kDa in size and located to the mitochondrial matrix.[1] Several natural variants in amino acid sequences exist. The structure of the MCEE protein has been resolved by X-ray crystallography[2] at 1.8-angstrom resolution.
Function
The MCEE gene encodes an enzyme that interconverts D- and L- methylmalonyl-CoA during the degradation of branched-chain amino acids, odd chain-length fatty acids, and other metabolites. In biochemistry terms, it catalyzes the reaction that converts (S)-methylmalonyl-CoA to the (R) form.[3][4] This enzyme catalyses the following chemical reaction
- (S)-methylmalonyl-CoA (R)-methylmalonyl-CoA
Methylmalonyl CoA epimerase plays an important role in the catabolism of fatty acids with odd-length carbon chains. In the catabolism of even-chain saturated fatty acids, the β-oxidation pathway breaks down fatty acyl-CoA molecules in repeated sequences of four reactions to yield one acetyl CoA per repeated sequence. This means that, for each round of β-oxidation, the fatty acyl-Co-A is shortened by two carbons. If the fatty acid began with an even number of carbons, this process could break down an entire saturated fatty acid into acetyl-CoA units. If the fatty acid began with an odd number of carbons, however, β-oxidation would break the fatty acyl-CoA down until the three carbon propionyl-CoA is formed. In order to convert this to the metabolically useful succinyl-CoA, three reactions are needed. The propionyl-CoA is first carboxylated to (S)-methylmalonyl-CoA by the enzyme Propionyl-CoA carboxylase. Methylmalonyl CoA epimerase then catalyzes the rearrangement of (S)-methylmalonyl-CoA to the (R) form in a reaction that uses a vitamin B12 cofactor and a resonance-stabilized carbanion intermediate. The (R)-methylmalonyl-CoA is then converted to succinyl-CoA in a reaction catalyzed by methylmalonyl-CoA mutase.
Acting as a general base, the enzyme abstracts a proton from the β-carbon of (R)-methylmalonyl-CoA. This results in the formation of a carbanion intermediate in which the α-carbon is stabilized by resonance. The enzyme then acts as a general acid to protonate the β-carbon, resulting in the formation of (S)-methylmalonyl-CoA.
Clinical significance
Mutations in the MCEE gene causes methymalonyl-CoA epimerase deficiency (MCEED),[5] a rare autosomal recessive inborn error of metabolism in amino acid metabolisms involving branched-chain amino acids valine, leucine, and isoleucine. Patients with MCEED may present with life-threatening neonatal metabolic acidosis, hyperammonemia, feeding difficulties, and coma.
References
- ↑ "MCEE - Methylmalonyl-CoA epimerase, mitochondrial precursor - Homo sapiens (Human) - MCEE gene & protein". www.uniprot.org.
- ↑ Europe, Protein Data Bank in. "PDB 3rmu structure summary ‹ Protein Data Bank in Europe (PDBe) ‹ EMBL-EBI". www.ebi.ac.uk.
- ↑ Mazumder R, Sasakawa T, Kaziro Y, Ochoa S (October 1962). "Metabolism of propionic acid in animal tissues. IX. Methylmalonyl coenzyme A racemase". The Journal of Biological Chemistry. 237 (10): 3065–8. doi:10.1016/S0021-9258(18)50121-6. PMID 13934211.
- ↑ Overath P, Kellerman GM, Lynen F, Fritz HP, Keller HJ (1962). "[On the mechanism of the rearrangement of methylmalonyl-Co A into succinyl-Co A. II. Experiments on the mechanism of action of methylmalonyl-Co A isomerase and methylmalonyl-Co A racemase]". Biochemische Zeitschrift. 335: 500–18. PMID 14482843.
- ↑ Bikker H, Bakker HD, Abeling NG, Poll-The BT, Kleijer WJ, Rosenblatt DS, Waterham HR, Wanders RJ, Duran M (July 2006). "A homozygous nonsense mutation in the methylmalonyl-CoA epimerase gene (MCEE) results in mild methylmalonic aciduria". Human Mutation. 27 (7): 640–3. doi:10.1002/humu.20373. PMID 16752391. S2CID 5821956.
External links
- methylmalonyl-CoA+epimerase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)