| |||
| Names | |||
|---|---|---|---|
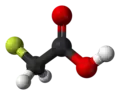
| Preferred IUPAC name
Fluoroacetic acid | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 3DMet | |||
| 1739053 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.120 | ||
| EC Number |
| ||
| 25730 | |||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2642 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H3FO2 | |||
| Molar mass | 78.042 g·mol−1 | ||
| Appearance | White solid | ||
| Density | 1.369 | ||
| Melting point | 35.2 °C (95.4 °F; 308.3 K) | ||
| Boiling point | 165 °C (329 °F; 438 K) | ||
| Soluble in water and ethanol | |||
| Acidity (pKa) | 2.586 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
Highly toxic and corrosive | ||
| GHS labelling: | |||
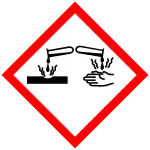  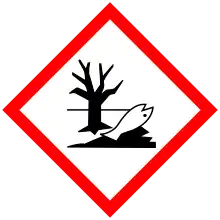 | |||
| Danger | |||
| H300, H314, H400 | |||
| P260, P264, P270, P273, P280, P301+P310, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P330, P363, P391, P405, P501 | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
7 mg/kg (rat, oral)[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Fluoroacetic acid is a organofluorine compound with formula CH2FCO2H. It is a colorless solid that is noted for its relatively high toxicity.[3] The conjugate base, fluoroacetate occurs naturally in at least 40 plants in Australia, Brazil, and Africa. It is one of only five known organic fluorine-containing natural products.[4]
Toxicity
Fluoroacetic acid is a harmful metabolite of some fluorine-containing drugs (median lethal dose, LD50 = 10 mg/kg in humans). The most common metabolic sources of fluoroacetic acid are fluoroamines and fluoroethers. Fluoroacetic acid can disrupt the Krebs cycle.[5]
In contrast with monofluoroacetic acid, difluoroacetic acid and trifluoroacetic acid are far less toxic. Its pKa is 2.66, in contrast to 1.24 and 0.23 for the respective di- and trifluorinated acids.[6]
Uses
Fluoroacetic acid is used to manufacture pesticides especially rodenticides (see sodium fluoroacetate). The overall market is projected to rise at a considerable rate during the forecast period, 2021 to 2027.[7]
See also
References
- ↑ as anion of fluoroacetic acid
- ↑ Fluoroacetic acid toxicity
- ↑ Timperley, Christopher M. (2000). "Highly-toxic fluorine compounds". Fluorine Chemistry at the Millennium. pp. 499–538. doi:10.1016/B978-008043405-6/50040-2. ISBN 9780080434056.
- ↑ K.K. Jason Chan; David O'Hagan (2012). The Rare Fluorinated Natural Products and Biotechnological Prospects for Fluorine Enzymology. Methods in Enzymology. Vol. 516. pp. 219–235. doi:10.1016/B978-0-12-394291-3.00003-4. ISBN 9780123942913. PMID 23034231.
- ↑ Kyzer, Jillian L.; Martens, Marvin (15 March 2021). "Metabolism and Toxicity of Fluorine Compounds". Chemical Research in Toxicology. 34 (3): 678–680. doi:10.1021/acs.chemrestox.0c00439. PMC 8023797. PMID 33513303.
- ↑ G. Siegemund; W. Schwertfeger; A. Feiring; B. Smart; F. Behr; H. Vogel; B. McKusick. "Fluorine Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_349. ISBN 978-3527306732.
- ↑ Industry Research (October 25, 2021). "Global Fluoroacetic Acid Market Share, Size 2021: Consumption Analysis By Applications, Future Demand, Top Leading Players, Competitive Situation and Emerging Trends, and Forecast to 2027". MarketWatch. Retrieved 5 January 2022.