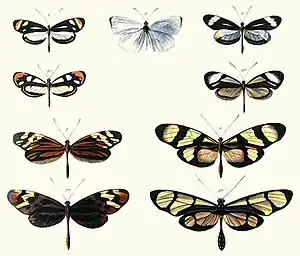
Müllerian mimicry is a natural phenomenon in which two or more well-defended species, often foul-tasting and sharing common predators, have come to mimic each other's honest warning signals, to their mutual benefit. The benefit to Müllerian mimics is that predators only need one unpleasant encounter with one member of a set of Müllerian mimics, and thereafter avoid all similar coloration, whether or not it belongs to the same species as the initial encounter. It is named after the German naturalist Fritz Müller, who first proposed the concept in 1878, supporting his theory with the first mathematical model of frequency-dependent selection, one of the first such models anywhere in biology.[lower-alpha 1][2][3]
Müllerian mimicry was first identified in tropical butterflies that shared colourful wing patterns, but it is found in many groups of insects such as bumblebees, and other animals such as poison frogs and coral snakes. The mimicry need not be visual; for example, many snakes share auditory warning signals. Similarly, the defences involved are not limited to toxicity; anything that tends to deter predators, such as foul taste, sharp spines, or defensive behaviour can make a species unprofitable enough to predators to allow Müllerian mimicry to develop.
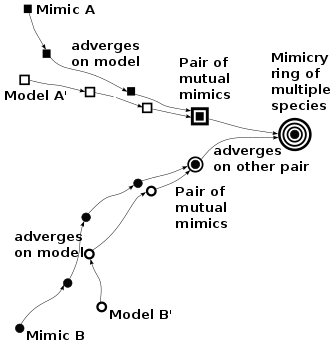
Once a pair of Müllerian mimics has formed, other mimics may join them by advergent evolution (one species changing to conform to the appearance of the pair, rather than mutual convergence), forming mimicry rings. Large rings are found for example in velvet ants. Since the frequency of mimics is positively correlated with survivability, rarer mimics are likely to adapt to resemble commoner models, favouring both advergence and larger Müllerian mimicry rings. Where mimics are not strongly protected by venom or other defences, honest Müllerian mimicry becomes, by degrees, the better-known bluffing of Batesian mimicry.
History
Origins

Müllerian mimicry was proposed by the German zoologist and naturalist Fritz Müller (1821–1897). An early proponent of evolution, Müller offered the first explanation for resemblance between certain butterflies that had puzzled the English naturalist Henry Walter Bates in 1862. Bates, like Müller, spent a significant part of his life in Brazil, as described in his book The Naturalist on the River Amazons. Bates conjectured that these abundant and distasteful butterflies might have been caused to resemble each other by their physical environment. Müller had also seen these butterflies first hand, and like Bates had collected specimens, and he proposed a variety of other explanations. One was sexual selection, namely that individuals would choose to mate with partners with frequently-seen coloration, such as those resembling other species. However, if as is usual, females are the choosers, then mimicry would be seen in males, but in sexually dimorphic species, females are more often mimetic.[5] Another was, as Müller wrote in 1878, that "defended species may evolve a similar appearance so as to share the costs of predator education."[6][7]
Müller's mathematical model
Müller's 1879 account was one of the earliest uses of a mathematical model in evolutionary ecology, and the first exact model of frequency-dependent selection.[8][9] Mallet calls Müller's mathematical assumption behind the model "beguilingly simple".[10] Müller presumed that the predators had to attack n unprofitable prey in a summer to experience and learn their warning coloration. Calling a1 and a2 the total numbers of two unprofitable prey species, Müller then argued that, if the species are completely unalike they each lose n individuals. However, if they resemble each other,[8]
then species 1 loses a1n/a1+a2 individuals, and species 2 loses a2n/a1+a2 individuals.
Species 1 therefore gains n-a1n/a1+a2 = a2n/a1+a2 and species 2 similarly gains a1n/a1+a2 in absolute numbers of individuals not killed.
The proportional gain compared to the total population of species 1 is g1 = a2n/a1(a1+a2) and similarly for species 2 g2 = a1n/a2(a1+a2), giving the per head fitness gain of the mimicry when the predators have been fully educated.
Hence, Müller concluded, the proportion g1:g2 was a2/a1 : a1/a2, which equals a22:a12, and the rarer species gains far more than the commoner one.[8]
The model is an approximation, and assumes the species are equally unprofitable. If one is more distasteful than the other, then the relative gains differ further, the less distasteful species benefiting more (as a square of the relative distastefulness) from the protection afforded by mimicry. This can be thought of as parasitic or quasi-Batesian, the mimic benefiting at the expense of the model. Later models are more complex and take factors such as rarity into account. The assumption of a fixed number n to be attacked is questionable.[5] Müller also effectively assumed a step function, when a gradual change (a functional response[11]) is more plausible.[10]
Non-deceitful mimicry
Biologists have not always viewed the Müllerian mechanism as mimicry, both because the term was strongly associated with Batesian mimicry, and because no deceit was involved—unlike the situation in Batesian mimicry, the aposematic signals given by Müllerian mimics are (unconsciously) honest. Earlier terms, no longer in use, for Müllerian mimicry included "homotypy", "nondeceitful homotypy" and "arithmetic homotypy".[12]
Evolution
Aposematism, camouflage, and mimicry
Müllerian mimicry relies on aposematism, or warning signals. Dangerous organisms with these honest signals are avoided by predators, which quickly learn after a bad experience not to pursue the same unprofitable prey again. Learning is not actually necessary for animals which instinctively avoid certain prey;[13] however, learning from experience is more common.[14] The underlying concept with predators that learn is that the warning signal makes the harmful organism easier to remember than if it remained as well camouflaged as possible. Aposematism and camouflage are in this way opposing concepts, but this does not mean they are mutually exclusive. Many animals remain inconspicuous until threatened, then suddenly employ warning signals, such as startling eyespots, bright colours on their undersides or loud vocalizations. In this way, they enjoy the best of both strategies. These strategies may also be employed differentially throughout development. For instance, large white butterflies are aposematic as larvae, but are Müllerian mimics once they emerge from development as adult butterflies.[15]
Selective advantage
Many different prey of the same predator could all employ their own warning signals, but this would make no sense for any party. If they could all agree on a common warning signal, the predator would have fewer detrimental experiences, and the prey would lose fewer individuals educating it. No such conference needs to take place, as a prey species that just so happens to look a little like an unprofitable[lower-alpha 2] species will be safer than its conspecifics, enabling natural selection to drive the prey species toward a single warning language. This can lead to the evolution of both Batesian and Müllerian mimicry, depending on whether the mimic is itself unprofitable to its predators, or just a free-rider. Multiple species can join the protective cooperative, expanding the mimicry ring. Müller thus provided an explanation for Bates' paradox; the mimicry was not, in his view, a case of exploitation by one species, but rather a mutualistic arrangement, though his mathematical model indicated a pronounced asymmetry.[7][16][9]
Relationship to Batesian mimicry
The Müllerian strategy is usually contrasted with Batesian mimicry, in which one harmless species adopts the appearance of an unprofitable species to gain the advantage of predators' avoidance; Batesian mimicry is thus in a sense parasitic on the model's defences, whereas Müllerian is to mutual benefit. However, because comimics may have differing degrees of protection, the distinction between Müllerian and Batesian mimicry is not absolute, and there can be said to be a spectrum between the two forms.[17]
Viceroy butterflies and monarchs (types of admiral butterfly) are both poisonous Müllerian mimics, though they were long thought to be Batesian. Mitochondrial DNA analysis of admiral butterflies shows that the viceroy is the basal lineage of two western sister species in North America. The variation in wing patterns appears to have preceded the evolution of toxicity, while other species remain non-toxic, refuting the hypothesis that the toxicity of these butterflies is a conserved characteristic from a common ancestor.[18]
Non-visual mimicry
Müllerian mimicry need not involve visual mimicry; it may employ any of the senses. For example, many snakes share the same auditory warning signals, forming an auditory Müllerian mimicry ring. More than one signal may be shared: snakes can make use of both auditory signals and warning coloration.[19]
Negative frequency-dependent selection
There is a negative correlation between the frequency of mimics and the "survivability" of both species involved. This implies that it is reproductively beneficial for both species if the models outnumber the mimics; this increases the negative interactions between predator and prey.[19]
Genetics
Some insight into the evolution of mimetic color mimicry in Lepidoptera in particular can be seen through the study of the Optix gene. The Optix gene is responsible for the Heliconius butterflies' signature red wing patterns that help it signal to predators that it is toxic. By sharing this coloration with other poisonous red winged butterflies the predator may have pursued previously, the Heliconius butterfly increases its chance of survival through association. By mapping the genome of many related species of Heliconius butterflies "show[s] that the cis-regulatory evolution of a single transcription factor can repeatedly drive the convergent evolution of complex color patterns in distantly related species…".[20] This suggests that the evolution of a non-coding piece of DNA that regulates the transcription of nearby genes can be the reason behind similar phenotypic coloration between distant species, making it hard to determine if the trait is homologous or simply the result of convergent evolution.
Two step evolution
One proposed mechanism for Müllerian mimicry is the "two step hypothesis". This states that a large mutational leap initially establishes an approximate resemblance of the mimic to the model, both species already being aposematic. In a second step, smaller changes establish a closer resemblance. This is only likely to work, however, when a trait is governed by a single gene, and many coloration patterns are certainly controlled by multiple genes.[21]
Advergence versus mutualism
The mimic poison frog Ranitomeya (Dendrobates) imitator is polymorphic, with a striped morph that imitates the black and yellow striped morph of Ranitomeya variabilis, a spotted morph that imitates the largely blue-green highland spotted morph also of R. variabilis, and a banded morph that imitates the red and black banded Ranitomeya summersi.[5][23]

R. imitator has thus apparently evolved in separate populations to resemble different targets, i.e. it has changed to resemble (adverged on) those target species, rather than both R. imitator and the other species mutually converging in the way that Müller supposed for tropical butterflies.[24]
Such advergence may be common. The mechanism was proposed by the entomologist F. A. Dixey in 1909[25] and has remained controversial; the evolutionary biologist James Mallet, reviewing the situation in 2001, suggested that in Müllerian mimicry, advergence may be more common than convergence. In advergent evolution, the mimicking species responds to predation by coming to resemble the model more and more closely. Any initial benefit is thus to the mimic, and there is no implied mutualism, as there would be with Müller's original convergence theory. However, once model and mimic have become closely similar, some degree of mutual protection becomes likely.[9][24] This theory would predict that all mimicking species in an area should converge on a single pattern of coloration. This does not appear to happen in nature, however, as Heliconius butterflies form multiple Müllerian mimicry rings in a single geographical area. The finding implies that additional evolutionary forces are probably at work.[22]
Mimicry complexes
- Many familiar bumblebees are Müllerian mimics, with effective stings and similar warning coloration
_-_queen_-_Flickr_-_S._Rae.jpg.webp)


_(34742598795).jpg.webp)
Müllerian mimicry often occurs in clusters of multiple species called rings. Müllerian mimicry is not limited to butterflies, where rings are common; mimicry rings occur among Hymenoptera, such as bumblebees, and other insects, and among vertebrates including fish and coral snakes. Bumblebees Bombus are all aposematically coloured in combinations, often stripes, of black, white, yellow, and red; and all their females have stings,[lower-alpha 3] so they are certainly unprofitable to predators. There is evidence that several species of bumblebees in each of several areas of the world, namely the American West and East coasts, Western Europe, and Kashmir, have converged or adverged on mutually mimetic coloration patterns. Each of these areas has one to four mimicry rings, with patterns different from those in other areas.[9]
(Id_%253F)_(16821757352).jpg.webp)
The relationships among mimics can become complex. For example, the poison fangblenny Meiacanthus spp. have hollow canines and poison glands, and are avoided by predatory fish. The blenny Plagiotremus townsendi resembles Meiacanthus and is eaten by a variety of predators, so it is a Batesian mimic in their case: but it is avoided by the lionfish, Pterois volitans, making it also a Müllerian mimic.[26]
Sets of associated rings are called complexes. Large complexes are known among the North American velvet ants in the genus Dasymutilla. Out of 351 species examined in one study, 336 had morphological similarities, apparently forming 8 distinct mimetic rings; 65 species in another study appeared to form six rings separable by both morphology and geography.[27][28]
Taxonomic range

Müllerian mimicry was discovered and has mainly been researched in insects. However, there is no reason why the mechanism's evolutionary advantages should not be exploited in other groups. There is some evidence that birds in the New Guinea genus Pitohui are Müllerian mimics. Pitohui dichrous and Pitohui kirhocephalus "share a nearly identical colour pattern" where their geographic ranges overlap, but differ elsewhere; they are conspicuous; and they are chemically defended by a powerful neurotoxic alkaloid, batrachotoxin, in their feathers and skin. This combination of facts implies that the populations in these zones of overlap have converged to share honest warning signals.[29]
Many species of flowers resemble each other but actual mimicry has not been demonstrated.[31] It has been proposed that spiny plants such as Cactaceae and Agave in the Americas, Aloe, Euphorbia, white-thorned Acacia in Africa and spiny Asteraceae of the Mediterranean may form Müllerian mimicry rings, as they are strongly defended, are generally agreed to be aposematic, have similar conspicuous patterns and coloration, and are found in overlapping territories.[32]
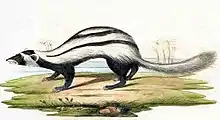
Aposematic mammals in the families Mustelidae, Viverridae, and Herpestidae have independently evolved conspicuous black-and-white coloration, suggesting that Müllerian mimicry may be involved.[30]
In marketing
The evolutionary zoologist Thomas N. Sherratt suggests that different types of mimicry occur in brand and product marketing. He notes that distinctive forms like the Coca-Cola bottle's shape are defended by businesses, whereas rival companies have often imitated such famous motifs to benefit from the investment and reputation of their well-known competitors, constituting Batesian mimicry. Sherratt observes that the packaging of British supermarket own brands of potato crisps are consistently colour-coded red for the ready-salted variety, green for salt and vinegar, and blue for cheese and onion,[lower-alpha 4] across the major chains Sainsbury's, Tesco, Asda, and Waitrose. He argues that this sharing of pattern is very unlikely to have arisen by chance, in which case the resemblance is intentionally to inform customers reliably (honest signalling) of what each package contains, to mutual benefit in the manner of Müllerian mimicry.[5]
See also
Notes
- ↑ Thomas Malthus's use of tables of numbers illustrating the limits to human population growth is one of the few earlier uses of a mathematical argument that could be called a model.
- ↑ Unprofitability may consist of anything which makes prey not worth a predator's while to eat. Unpalatability on grounds of toxicity or foul taste is a common mechanism, but defences may include sharp spines; an aggressive nature; agility or speed in escape rendering the prey costly to catch; foul smell, and so on.[9]
- ↑ Drones have no sting, but similar patterns, and may (more or less accidentally) benefit from automimicry of females of their own species.[9]
- ↑ Sherratt notes that this use of red is shared with Walker's crisps, whereas the uses of blue and green are interchanged with respect to Walker's.[5]
References
- ↑ Meyer, A. (2006). "Repeating patterns of mimicry". PLOS Biol. 4 (10): e341. doi:10.1371/journal.pbio.0040341. PMC 1617347. PMID 17048984.
- ↑ Müller, Fritz (1878). "Ueber die Vortheile der Mimicry bei Schmetterlingen". Zoologischer Anzeiger. 1: 54–55.
- ↑ Müller, Fritz (1879). "Ituna and Thyridia; a remarkable case of mimicry in butterflies. (R. Meldola translation)". Proclamations of the Entomological Society of London. 1879: 20–29.
- ↑ Ritland, D.; L. P. Brower (1991). "The viceroy butterfly is not a Batesian mimic". Nature. 350 (6318): 497–498. Bibcode:1991Natur.350..497R. doi:10.1038/350497a0. S2CID 28667520.
Viceroys are as unpalatable as monarchs, and significantly more unpalatable than queens from representative Florida populations.
- 1 2 3 4 5 Sherratt, T. (2008). "The Evolution of Müllerian Mimicry". Die Naturwissenschaften. 95 (8): 681–695. Bibcode:2008NW.....95..681S. doi:10.1007/s00114-008-0403-y. PMC 2443389. PMID 18542902.
- ↑ Forbes 2009, pp. 40–42.
- 1 2 Ruxton, Speed & Sherratt 2004, pp. 116–118.
- 1 2 3 "Fritz Müller in 1891". Retrieved 18 November 2017.
- 1 2 3 4 5 6 Mallet, James (2001). "Causes and consequences of a lack of coevolution in Mullerian mimicry". Evolutionary Ecology. 13 (7–8): 777–806. CiteSeerX 10.1.1.508.2755. doi:10.1023/a:1011060330515. S2CID 40597409.
- 1 2 Mallet, James (July 2001). "Mimicry: An interface between psychology and evolution". PNAS. 98 (16): 8928–8930. Bibcode:2001PNAS...98.8928M. doi:10.1073/pnas.171326298. PMC 55348. PMID 11481461.
- ↑ Holling, C. S. (May 1959). "The components of predation as revealed by a study of small-mammal predation of the European pine sawfly". The Canadian Entomologist. 91 (5): 293–320. doi:10.4039/Ent91293-5. S2CID 53474917.
- ↑ Pasteur, G. (1982). "A Classificatory Review of Mimicry". Annual Review of Ecology and Systematics. 13 (1): 169–199. doi:10.1146/annurev.es.13.110182.001125.
- ↑ Smith, S. M. (1975). "Innate Recognition of Coral Snake Pattern by a Possible Avian Predator". Science. 187 (4178): 759–760. Bibcode:1975Sci...187..759S. doi:10.1126/science.187.4178.759. PMID 17795249. S2CID 41092574.
- ↑ Wickler, Wolfgang (1998). "Mimicry". Encyclopædia Britannica. Vol. 24 (15th ed.). pp. 144–151.
- ↑ Feltwell, John (1982). Large White Butterfly: The Biology, Biochemistry, and Physiology of Pieris Brassicae (Linnaeus). The Hague: W. Junk. ISBN 978-90-6193-128-7.
- ↑ Ruxton, Speed & Sherratt 2004, p. 126.
- ↑ Brower, L. P.; Ryerson, W. N.; Coppinger, L. L.; Glazier, S. C. (1968). "Ecological chemistry and the palatability spectrum". Science. 161 (3848): 1349–51. Bibcode:1968Sci...161.1349B. doi:10.1126/science.161.3848.1349. PMID 17831347. S2CID 45185502.
- ↑ Mullen, S.P. (2006). "Wing pattern evolution and the origins of mimicry among North American admiral butterflies (Nymphalidae: Limenitis)". Molecular Phylogenetics and Evolution. 39 (3): 747–758. doi:10.1016/j.ympev.2006.01.021. PMID 16500119.
- 1 2 Ihalainen, E.; Lindstrèom, L.; Mappes, J.; Puolakkainen, S. (2008). "Butterfly effects in mimicry? Combining signal and taste can twist the relationship of Müllerian co-mimics". Behavioral Ecology and Sociobiology. 62 (8): 1267–1276. doi:10.1007/s00265-008-0555-y. S2CID 21823655.
- ↑ Reed, R.D.; Papa, R.; Martin, A.; Hines, H.M.; Counterman, B.A.; Pardo-Diaz, C.; Jiggins, C.D.; McMillan, W. (2011). "Optics drives the repeated convergent evolution of butterfly wing pattern mimicry". Science. 333 (6046): 1137–1141. Bibcode:2011Sci...333.1137R. doi:10.1126/science.1208227. PMID 21778360. S2CID 206535158.
- ↑ Balogh, A.; et al. (2009). "Feature Theory and the Two-step Hypothesis of Müllerian Mimicry Evolution". International Journal of Organic Evolution. 64 (3): 810–22. doi:10.1111/j.1558-5646.2009.00852.x. PMID 19796146. S2CID 205782455.
- 1 2 Mallet, James; Gilbert, Lawrence E. (1995). "Why are there so many mimicry rings? Correlations between habitat, behaviour and mimicry in Heliconius butterflies" (PDF). Biological Journal of the Linnean Society. 55 (2): 159–180. doi:10.1111/j.1095-8312.1995.tb01057.x.
- ↑ Schulte, Rainer (1989). "Dendrobates imitator. Eine Neue Dendrobates-Art aus Ostperu (Amphibia: Salentia: Dendrobatidae)". Sauria (in German). 8 (3): 11–20.
- 1 2 Ruxton, Speed & Sherratt 2004, pp. 126–127.
- ↑ Dixey, F. A. (1909). "On Müllerian mimicry and diaposematism. A reply to Mr G. A. K. Marshall". Transactions of the Entomological Society of London. 23: 559–583.
- ↑ Edmunds 1974, pp. 127–130.
- ↑ Wilson, Joshua S.; Williams, Kevin A.; Forister, Matthew L.; von Dohlen, Carol D.; Pitts, James P. (2012). "Repeated evolution in overlapping mimicry rings among north American velvet ants". Nature Communications. 3: 1272. Bibcode:2012NatCo...3.1272W. doi:10.1038/ncomms2275. PMID 23232402.
- ↑ Wilson, Joshua S.; Jahner, Joshua P.; Forister, Matthew L.; Sheehan, Erica S.; Williams, Kevin A.; Pitts, James P. (2015). "North American velvet ants form one of the world's largest known Müllerian mimicry complexes" (PDF). Current Biology. 25 (16): R704–R7064. doi:10.1016/j.cub.2015.06.053. PMID 26294178. S2CID 49367257.
- ↑ Dumbacher, J. P.; Fleischer, R. C. (2001). "Phylogenetic evidence for colour pattern convergence in toxic pitohuis: Mullerian mimicry in birds?". Proceedings of the Royal Society B: Biological Sciences. 268 (1480): 1971–1976. doi:10.1098/rspb.2001.1717. PMC 1088837. PMID 11571042.
- 1 2 Caro, Tim (2005). Antipredator Defenses in Birds and Mammals. University of Chicago Press. pp. 242–244. ISBN 978-0226094366.
- ↑ Roy, B. (1999). "Floral mimicry: a fascinating yet poorly understood phenomenon". Trends in Plant Science. 4 (8): 325–330. doi:10.1016/S1360-1385(99)01445-4. PMID 10431223.
- ↑ Lev-Yadun, Simcha (2014). "Müllerian mimicry in aposematic spiny plants". Plant Signaling & Behavior. 4 (6): 482–483. doi:10.4161/psb.4.6.8848. PMC 2688291. PMID 19816137.
Sources
- Edmunds, M. (1974). Defence in Animals. Longmans. ISBN 978-0-582-44132-3.
- Forbes, Peter (2009). Dazzled and Deceived: Mimicry and Camouflage. Yale University Press. ISBN 978-0-300-17896-8.
- Ruxton, Graeme; Speed, M. P.; Sherratt, T. N. (2004). Avoiding Attack. The Evolutionary Ecology of Crypsis, Warning Signals and Mimicry. Oxford University Press. ISBN 978-0-19-852860-9. Chapters 9 and 11 provide an overview.
Further reading
- Wickler, Wolfgang (1968). Mimicry in Plants and Animals. McGraw-Hill. ISBN 978-0-07-070100-7. Especially chapters 7 and 8.