| Mycoplasma pneumoniae | |
|---|---|
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Mycoplasmatota |
| Class: | Mollicutes |
| Order: | Mycoplasmatales |
| Family: | Mycoplasmataceae |
| Genus: | Mycoplasma |
| Species: | M. pneumoniae |
| Binomial name | |
| Mycoplasma pneumoniae Somerson et al., 1963 | |
Mycoplasma pneumoniae is a very small bacterium in the class Mollicutes. It is a human pathogen that causes the disease mycoplasma pneumonia, a form of atypical bacterial pneumonia related to cold agglutinin disease. M. pneumoniae is characterized by the absence of a peptidoglycan cell wall and resulting resistance to many antibacterial agents. The persistence of M. pneumoniae infections even after treatment is associated with its ability to mimic host cell surface composition.
Discovery and history
In 1898, Nocard and Roux isolated an agent assumed to be the cause of cattle pneumonia and named it microbe de la peripneumonie[1][2][3][4][5][6] Microorganisms from other sources, having properties similar to the pleuropneumonia organism (PPO) of cattle, soon came to be known as pleuropneumonia-like organisms (PPLO), but their true nature remained unknown.[1][2][3][4] Many PPLO were later proven to be the cause of pneumonias and arthritis in several lower animals.[1][7][8][9]
In 1944, Monroe Eaton used embryonated chicken eggs to cultivate an agent thought to be the cause of human primary atypical pneumonia (PAP), commonly known as "walking pneumonia."[10] This unknown organism became known as the "Eaton agent".[11] At that time, Eaton's use of embryonated eggs, then used for cultivating viruses, supported the idea that the Eaton agent was a virus. Yet it was known that PAP was amenable to treatment with broad-spectrum antibiotics, making a viral etiology suspect.[1][2][7][12][13]
Robert Chanock, a researcher from the NIH who was studying the Eaton agent as a virus, visited the Wistar Institute in Philadelphia in 1961 to obtain a cell culture of a normal human cell strain developed by Leonard Hayflick. This cell strain was known to be exquisitely sensitive to isolate and grow human viruses. Chanock told Hayflick of his research on the Eaton agent, and his belief that its viral nature was questionable. Although Hayflick knew little about the current research on this agent, his doctoral dissertation had been done on animal diseases caused by PPLO. Hayflick knew that many lower animals suffered from pneumonias caused by PPLOs (later to be termed mycoplasmas). Hayflick reasoned that the Eaton agent might be a mycoplasma, and not a virus. Chanock had never heard of mycoplasmas, and at Hayflick's request sent him egg yolk containing the Eaton agent.[1][4][14][15][16][17]
Using a novel agar and fluid medium formulation he had devised,[14] Hayflick isolated a unique mycoplasma from the egg yolk. This was soon proven by Chanock and Hayflick to be the causative agent of PAP.[14][18][19][20] When this discovery became known to Emmy Klieneberger-Nobel of the Lister Institute in London, the world's leading authority on these organisms, she suggested that the organism be named Mycoplasma hayflickiae.[21] Hayflick demurred in favor of Mycoplasma pneumoniae.[22][23]
This smallest free-living microorganism was the first to be isolated and proven to be the cause of a human disease. For his discovery, Hayflick was presented with the Presidential Award by the International Organization of Mycoplasmology. The inverted microscope under which Hayflick discovered Mycoplasma pneumoniae is kept by the Smithsonian Institution.[20]
Taxonomy and classification
The term mycoplasma (mykes meaning fungus, and plasma, meaning formed) is derived from the fungal-like growth of some mycoplasma species.[6] The mycoplasmas were classified as Mollicutes (“mollis”, meaning soft and “cutis”, meaning skin) in 1960 due to their small size and genome, lack of cell wall, low G+C content and unusual nutritional needs.[6][24] M. pneumoniae has also been designated as an arginine nonfermenting species.[25] Mycoplasmas are further classified by the sequence composition of 16s rRNA. All mycoplasmas of the pneumoniae group possess similar 16s rRNA variations unique to the group, of which M. pneumoniae has a 6.3% variation in the conserved regions, that suggest mycoplasmas formed by degenerative evolution from the gram-positive eubacterial group that includes bacilli, streptococci, and lactobacilli.[6][24][25] M. pneumoniae is a member of the family Mycoplasmataceae and order Mycoplasmatales.[6]
Cell biology

Mycoplasmas, which are among the smallest self-replicating organisms, are parasitic species that lack a cell wall and periplasmic space, have reduced genomes, and limited metabolic activity.[6][25][26] Mycoplasma pneumoniae cells have an elongated shape that is approximately 0.1–0.2 µm (100-200 nm) in width and 1-2 µm (1000-2000 nm) in length. The extremely small cell size means they are incapable of being examined by light microscopy; a stereomicroscope is required for viewing the morphology of M. pneumoniae colonies, which are usually less than 100 µm in length.[6] The inability to synthesize a peptidoglycan cell wall is due to the absence of genes encoding its formation and results in an increased importance in maintenance of osmotic stability to avoid desiccation.[6] The lack of a cell wall also calls for increased support of the cell membrane(reinforced with sterols), which includes a rigid cytoskeleton composed of an intricate protein network and, potentially, an extracellular capsule to facilitate adherence to the host cell.[6] M. pneumoniae are the only bacterial cells that possess cholesterol in their cell membrane (obtained from the host) and possess more genes that encode for membrane lipoprotein variations than other mycoplasmas,[25] which are thought to be associated with its parasitic lifestyle. M. pneumoniae cells also possess an attachment organelle, which is used in the gliding motility of the organism by an unknown mechanism.[6]
Genomics and metabolic reconstruction
Sequencing of the M. pneumoniae genome in 1996 revealed it is 816,394 bp in size.[24] The genome contains 687 genes that encode for proteins, of which about 56.6% code for essential metabolic enzymes; notably those involved in glycolysis and organic acid fermentation.[6][24][25][27] M. pneumoniae is consequently very susceptible to loss of enzymatic function by gene mutations, as the only buffering systems against functional loss by point mutations are for maintenance of the pentose phosphate pathway and nucleotide metabolism.[27] Loss of function in other pathways is suggested to be compensated by host cell metabolism.[27] In addition to the potential for loss of pathway function, the reduced genome of M. pneumoniae outright lacks a number of pathways, including the TCA cycle, respiratory electron transport chain, and biosynthesis pathways for amino acids, fatty acids, cholesterol and purines and pyrimidines.[6][25][27] These limitations make M. pneumoniae dependent upon import systems to acquire essential building blocks from their host or the environment that cannot be obtained through glycolytic pathways.[25][27] Along with energy costly protein and RNA production, a large portion of energy metabolism is exerted to maintain proton gradients (up to 80%) due to the high surface area to volume ratio of M. pneumoniae cells. Only 12 – 29% of energy metabolism is directed at cell growth, which is unusually low for bacterial cells, and is thought to be an adaptation of its parasitic lifestyle.[27]
Alternative genetic code
Unlike other bacteria, M. pneumoniae uses the codon UGA to code for tryptophan rather than using it as a stop codon.[6][24]
Comparative metabolomics
Mycoplasma pneumoniae has a reduced metabolome in comparison to other bacterial species.[28] This means that the pathogen has fewer metabolic reactions in comparison to other bacterial species such as B.subtilis and Escherichia coli.[28][29]
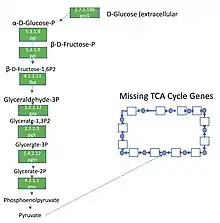
Since Mycoplasma pneumoniae has a reduced genome, it has a smaller number of overall paths and metabolic enzymes, which contributes to its more linear metabolome.[28] A linear metabolome causes Mycoplasma pneumoniae to be less adaptable to external factors.[28] Additionally, since Mycoplasma pneumoniae has a reduced genome, the majority of its metabolic enzymes are essential.[28] This is in contrast to another model organism, Escherichia coli, in which only 15% of its metabolic enzymes are essential.[28] In summary, the linear topology of Mycoplasma pneumoniae's metabolome leads to reduced efficiency in its metabolic reactions, but still maintains similar levels of metabolite concentrations, cellular energetics, adaptability, and global gene expression.[28]
| Species | M. pneumoniae | L. lactis | B. subtilis | E. coli |
|---|---|---|---|---|
| Mean # Of Paths | 8.17 | 5.37 | 7.54 | 6.12 |
The table above depicts the mean path length for the metabolomes of M. pneumoniae, E. coli, L. lactis, and B. subtilis.[28] This number describes, essentially, the mean number of reactions that occur in the metabolome. Mycoplasma pneumoniae, on average, has a high number of reactions per path within its metabolome in comparison to other model bacterial species.[28]
One effect of Mycoplasma pneumoniae’s unique metabolome is its longer duplication time.[28] It takes the pathogen significantly more time to duplicate on average compared to other model organism bacteria.[28] This may be due to the fact that Mycoplasma pneumoniae’s metabolome is less efficient than that of Escherichia coli.[28]
The metabolome of Mycoplasma pneumoniae can also be informative in analyzing its pathogenesis.[31] Extensive study of the metabolic network of this organism has led to the identification of biomarkers that can potentially reveal the presence of the extensive complications the bacteria can cause.[31] Metabolomics is increasingly being used as a useful tool for the verification of biomarkers of infectious pathogens.[31]
Host and reproduction
Mycoplasma pneumoniae grows exclusively by parasitizing mammals. Reproduction, therefore, is dependent upon attachment to a host cell. According to Waite and Talkington, specialized reproduction occurs by “binary fission, temporally linked with duplication of its attachment organelle, which migrates to the opposite pole of the cell during replication and before nucleoid separation”.[6] Mutations that affect the formation of the attachment organelle not only hinder motility and cell division, but also reduce the ability of M. pneumoniae cells to adhere to the host cell.[25]
Pathogenicity

Mycoplasma pneumoniae parasitizes the respiratory tract epithelium of humans.[6] Adherence to the respiratory epithelial cells is thought to occur via the attachment organelle, followed by evasion of host immune system by intracellular localization and adjustment of the cell membrane composition to mimic the host cell membrane.
Cytoadherence
Adherence of M. pneumoniae to a host cell (usually a respiratory tract cell, but occasionally an erythrocyte or urogenital lining cell) is the initiating event for pneumonic disease and related symptoms.[6] The specialized attachment organelle is a polar, electron dense and elongated cell extension that facilitates motility and adherence to host cells.[6][25] It is composed of a central filament surrounded by an intracytoplasmic space, along with a number of adhesins and structural and accessory proteins localized at the tip of the organelle.[6][25] A variety of proteins are known to contribute to the formation and functionality of the attachment organelle, including the accessory proteins HMW1–HMW5, P30, P56, and P90 that confer structure and adhesin support, and P1, P30 and P116 which are involved directly in attachment.[6][32][33] This network of proteins participates not only in the initiation of attachment organelle formation and adhesion but also in motility.[33] The P1 adhesin (trypsin-sensitive protein) is a 120 kDa protein highly clustered on the surface of the attachment organelle tip in virulent mycoplasmas.[6][33][34] Both the presence of P1 and its concentration on the cell surface are required for the attachment of M. pneumoniae to the host cell. M. pneumoniae cells treated with monoclonal antibodies specific to the immunogenic C-terminus of the P1 adhesin have been shown to be inhibited in their ability to attach to the host cell surface by approximately 75%, suggesting P1 is a major component in adherence.[6][32][33] These antibodies also decreased the ability of the cell to glide quickly, which may contribute to decreased adherence to the host by hindering their capacity to locate a host cell.[32] Furthermore, mutations in P1 or degradation by trypsin treatment yield avirulent M. pneumoniae cells.[6] Loss of proteins in the cytoskeleton involved in the localization of P1 in the tip structure, such as HMW1–HMW3, also cause avirulence due to the lack of adhesin clustering.[33][34] Another protein considered to play an important role in adherence is P30, as M. pneumoniae cells with mutations in this protein or that have had antibodies raised against P30 are incapable of adhering to host cells.[6][25] P30 is not involved in the localization of P1 in the tip structure since P1 is trafficked to the attachment organelle in P30 mutants, but rather it may function as a receptor-binding accessory adhesin.[25][34] P30 mutants also display distinct morphological features such as multiple lobes and a rounded shape as opposed to elongated, which suggests P30 may interact with the cytoskeleton during formation of the attachment organelle.[25] A number of eukaryotic cell surface components have been implicated in the adherence of M. pneumoniae cells to the respiratory tract epithelium. Among them are sialoglycoconjugates, sulfated glycolipids, glycoproteins, fibronectin, and neuraminic acid receptors.[6][32][35] Lectins on the surface of the bacterial cells are capable of binding oligosaccharide chains on glycolipids and glycoproteins to facilitate attachment, in addition to the proteins TU and pyruvate dehydrogenase E1 β, which bind to fibronectin.[6][32]

Intracellular localization
Mycoplasma pneumoniae is known to evade host immune system detection, resist antibiotic treatment, and cross mucosal barriers, which may be due to its ability to fuse with host cells and survive intracellularly.[6][26] In addition to the close physical proximity of M. pneumoniae and host cells, the lack of cell wall and peculiar cell membrane components, like cholesterol, may facilitate fusion (1). Internal localization may produce chronic or latent infections as M. pneumoniae is capable of persisting, synthesizing DNA, and replicating within the host cell even after treatment with antibiotics.[26] The exact mechanism of intracellular localization is unknown, however the potential for cytoplasmic sequestration within the host explains the difficulty in completely eliminating M. pneumoniae infections in afflicted individuals.[6]
Immune response
In addition to evasion of host immune system by intracellular localization, M. pneumoniae can change the composition of its cell membrane to mimic the host cell membrane and avoid detection by immune system cells. M. pneumoniae cells possess a number of protein and glycolipid antigens that elicit immune responses, but variation of these surface antigens would allow the infection to persist long enough for M. pneumoniae cells to fuse with host cells and escape detection. The similarity between the compositions of M. pneumoniae and human cell membranes can also result in autoimmune responses in several organs and tissues.[6]
Cytotoxicity and organismal effects
The main cytotoxic effect of M. pneumoniae is local disruption of tissue and cell structure along the respiratory tract epithelium due to its close proximity to host cells. Attachment of the bacteria to host cells can result in loss of cilia, a reduction in metabolism, biosynthesis, and import of macromolecules, and, eventually, infected cells may be shed from the epithelial lining.[6] M. pneumoniae produces a unique virulence factor known as Community Acquired Respiratory Distress Syndrome (CARDS) toxin.[36] The CARDS toxin most likely aids in the colonization and pathogenic pathways of M. pneumoniae, leading to inflammation and airway dysfunction. In addition, the formation of hydrogen peroxide is a key virulence factor in M. pneumoniae infections.[6] Attachment of M. pneumoniae to erythrocytes permits diffusion of hydrogen peroxide from the bacteria to the host cell without detoxification by catalase or peroxidase, which can injure the host cell by reducing glutathione, damaging lipid membranes and causing protein denaturation.[6][35] Local damage may also be a result of lactoferrin acquisition and subsequent hydroxyl radical, superoxide anion and peroxide formation.[6] The cytotoxic effects of M. pneumoniae infections translate into common symptoms like coughing and lung irritation that may persist for months after infection has subsided. Local inflammation and hyperresponsiveness by infection induced cytokine production has been associated with chronic conditions such as bronchial asthma and has also been linked to progression of symptoms in individuals with cystic fibrosis and COPD.[6]
See also
| External videos | |
|---|---|
References
- 1 2 3 4 5 Hayflick L, Chanock RM (June 1965). "Mycoplasma species of man". Bacteriological Reviews. 29 (2): 185–221. doi:10.1128/mmbr.29.2.185-221.1965. PMC 441270. PMID 14304038.
- 1 2 3 Hayflick, L. (May 1965). "The mycoplasma (PPLO) species of man". Transactions of the New York Academy of Sciences. Series II. 27 (7): 817–827. doi:10.1111/j.2164-0947.1965.tb02241.x. PMID 14333465.
- 1 2 Hayflick, L. (1967). Hayflick, L. (ed.). Biology of the mycoplasmas. Second Conference on the Biology of the Mycoplasmas. Vol. 143. Annals of the New York Academy of Sciences. pp. 5–6.
- 1 2 3 Hayflick, L., ed. (1969). The Mycoplasmatales and the L-phase of Bacteria. New York, NY: Appleton-Century-Crofts. ISBN 9780608123905.
- ↑ Marmion, B.P. (1990). "Eaton agent – science and scientific acceptance: A historical commentary". Reviews of Infectious Diseases. 12 (2): 338–353. doi:10.1093/clinids/12.2.338. PMID 2109871.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 Waites KB, Talkington DF (October 2004). "Mycoplasma pneumoniae and its role as a human pathogen". Clinical Microbiology Reviews. 17 (4): 697–728. doi:10.1128/CMR.17.4.697-728.2004. PMC 523564. PMID 15489344.
- 1 2 Razin S, Hayflick L (March 2010). "Highlights of mycoplasma research – an historical perspective". Biologicals. 38 (2): 183–190. doi:10.1016/j.biologicals.2009.11.008. PMID 20149687.
- ↑ Hayflick, L. (1956). The growth of human and poultry pleuropneumonia-like organisms in tissue cultures and in ovo, and the characterization of an infectious agent causing tendovaginitis with arthritis in chickens (Ph.D. thesis). University of Pennsylvania.
- ↑ Hayflick L, Stinebring WR (January 1960). "Intracellular growth of pleuropneumonialike organisms (PPLO) in tissue culture and in ovo". Annals of the New York Academy of Sciences. 79 (10): 433–449. Bibcode:1960NYASA..79..433H. doi:10.1111/j.1749-6632.1960.tb42709.x. PMID 14400338. S2CID 21089254.
- ↑ Eaton MD, Meiklejohn G, van Herick W (June 1944). "Studies on the etiology of primary atypical pneumonia : A filterable agent transmissible to cotton rats, hamsters, and chick embryos". The Journal of Experimental Medicine. 79 (6): 649–668. doi:10.1084/jem.79.6.649. PMC 2135382. PMID 19871393.
- ↑ Dajani AS, Clyde WA, Denny FW (June 1965). "Experimental infection with Mycoplasma pneumoniae (Eaton's agent)". The Journal of Experimental Medicine. 121 (6): 1071–1086. doi:10.1084/jem.121.6.1071. PMC 2138014. PMID 14319403.
- ↑ Hayflick, L. (1969). "Fundamental biology of the class Mollicutes, order Mycoplasmatales". In Hayflick, L. (ed.). The Mycoplasmatales and the L-phase of Bacteria. New York, NY: Appleton-Century-Crofts. ISBN 9780608123905.
- ↑ Hayflick, L. (1971). "Biology of the Mycoplasmatales". In Madoff, S. (ed.). Mycoplasmas and the L-forms of Bacteria. New York, NY: Gordon and Breach. doi:10.1002/jobm.19720120516.
- 1 2 3 Hayflick, L. (1965). "Tissue cultures and mycoplasmas". Texas Reports on Biology and Medicine. 23 (1): 285–303. PMID 5833547.
- ↑ Hayflick, L. (1966). "The role of mycoplasmas in human disease". The New Physician. December: 328–333, 348–350.
- ↑ Hayflick, L. (1972). Mycoplasmas as pathogens. CIBA Foundation Symposium: Pathogenic Mycoplasmas. Amsterdam, NL: Elsevier Excerpta Medica. pp. 17–31.
- ↑ Hayflick, L. (4 October 1993). "Isolation and identification of a mycoplasma as the etiological agent of primary atypical pneumonia in humans". Citation Classic. Current Contents. 40: 8.
- ↑ Chanock RM, Hayflick L, Barile MF (January 1962). "Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO". Proceedings of the National Academy of Sciences of the United States of America. 48 (1): 41–49. Bibcode:1962PNAS...48...41C. doi:10.1073/pnas.48.1.41. PMC 285494. PMID 13878126.
- 1 2 "Robert Chanock and the Eaton Agent". Web of Stories. 8 August 2012.
- 1 2 Sharrer, T. (2007). "Leitz inverted microscopes, Circa 1958". The Scientist. 21 (3): 96.
- ↑ Klieneberger-Nobel, E. (1980). Memoires (English ed.). London, UK: Academic Press. ISBN 0-12-414850-6.
- ↑ Chanock, R.M. (May 1963). "Mycoplasma pneumoniae: Proposed nomenclature for atypical pneumonia organism (Eaton agent)". Science. 140 (3567): 662. Bibcode:1963Sci...140..662C. doi:10.1126/science.140.3567.662. PMID 14020096. S2CID 34513645.
- ↑ Edward DG, Freundt EA, Chanock RM, Fabricant J, Hayflick L, Lemcke RM, et al. (March 1967). "Recommendations on nomenclature of the order Mycoplasmatales". Science. 155 (3770): 1694–1696. Bibcode:1967Sci...155.1694E. doi:10.1126/science.155.3770.1694. PMID 6020298.
- 1 2 3 4 5 Weisburg WG, Tully JG, Rose DL, Petzel JP, Oyaizu H, Yang D, et al. (December 1989). "A phylogenetic analysis of the mycoplasmas: basis for their classification". Journal of Bacteriology. 171 (12): 6455–67. doi:10.1128/jb.171.12.6455-6467.1989. PMC 210534. PMID 2592342.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Romero-Arroyo CE, Jordan J, Peacock SJ, Willby MJ, Farmer MA, Krause DC (February 1999). "Mycoplasma pneumoniae protein P30 is required for cytadherence and associated with proper cell development". Journal of Bacteriology. 181 (4): 1079–87. doi:10.1128/JB.181.4.1079-1087.1999. PMC 93483. PMID 9973332.
- 1 2 3 Dallo SF, Baseman JB (November 2000). "Intracellular DNA replication and long-term survival of pathogenic mycoplasmas". Microbial Pathogenesis. 29 (5): 301–9. doi:10.1006/mpat.2000.0395. PMID 11031124.
- 1 2 3 4 5 6 Wodke JA, Puchałka J, Lluch-Senar M, Marcos J, Yus E, Godinho M, et al. (2013). "Dissecting the energy metabolism in Mycoplasma pneumoniae through genome-scale metabolic modeling". Molecular Systems Biology. 9: 653. doi:10.1038/msb.2013.6. PMC 3658275. PMID 23549481.
- 1 2 3 4 5 6 7 8 9 10 11 12 Yus E, Maier T, Michalodimitrakis K, van Noort V, Yamada T, Chen WH, et al. (November 2009). "Impact of genome reduction on bacterial metabolism and its regulation". Science. 326 (5957): 1263–1268. Bibcode:2009Sci...326.1263Y. doi:10.1126/science.1177263. PMID 19965476. S2CID 17576843.
- ↑ Chowdhury S, Hepper S, Lodi MK, Saier MH, Uetz P (April 2021). "The Protein Interactome of Glycolysis in Escherichia coli". Proteomes. 9 (2): 16. doi:10.3390/proteomes9020016. PMC 8167557. PMID 33917325.
- ↑ "KEGG PATHWAY: Glycolysis / Gluconeogenesis - Mycoplasma pneumoniae 309". www.genome.jp. Retrieved 2022-10-27.
- 1 2 3 Li J, Luu LD, Wang X, Cui X, Huang X, Fu J, et al. (December 2022). "Metabolomic analysis reveals potential biomarkers and the underlying pathogenesis involved in Mycoplasma pneumoniae pneumonia". Emerging Microbes & Infections. 11 (1): 593–605. doi:10.1080/22221751.2022.2036582. PMC 8865114. PMID 35094669.
- 1 2 3 4 5 Drasbek M, Christiansen G, Drasbek KR, Holm A, Birkelund S (November 2007). "Interaction between the P1 protein of Mycoplasma pneumoniae and receptors on HEp-2 cells". Microbiology. 153 (Pt 11): 3791–3799. doi:10.1099/mic.0.2007/010736-0. PMID 17975088.
- 1 2 3 4 5 Baseman JB, Cole RM, Krause DC, Leith DK (September 1982). "Molecular basis for cytadsorption of Mycoplasma pneumoniae". Journal of Bacteriology. 151 (3): 1514–22. doi:10.1128/JB.151.3.1514-1522.1982. PMC 220433. PMID 6809731.
- 1 2 3 Hahn TW, Willby MJ, Krause DC (March 1998). "HMW1 is required for cytadhesin P1 trafficking to the attachment organelle in Mycoplasma pneumoniae". Journal of Bacteriology. 180 (5): 1270–6. doi:10.1128/JB.180.5.1270-1276.1998. PMC 107017. PMID 9495768.
- 1 2 Sobeslavsky O, Prescott B, Chanock RM (September 1968). "Adsorption of Mycoplasma pneumoniae to neuraminic acid receptors of various cells and possible role in virulence". Journal of Bacteriology. 96 (3): 695–705. doi:10.1128/JB.96.3.695-705.1968. PMC 252361. PMID 4183967.
- ↑ "CDC Mycoplasma Pneumoniae". CDC. Retrieved 23 September 2015.
This article incorporates public domain text from the CDC as cited.
Further reading
- Baseman JB, Reddy SP, Dallo SF (October 1996). "Interplay between mycoplasma surface proteins, airway cells, and the protean manifestations of mycoplasma-mediated human infections". American Journal of Respiratory and Critical Care Medicine. 154 (4 Pt 2): S137-44. doi:10.1164/ajrccm/154.4_Pt_2.S137. PMID 8876532.
- Razin S, Yogev D, Naot Y (December 1998). "Molecular biology and pathogenicity of mycoplasmas". Microbiology and Molecular Biology Reviews. 62 (4): 1094–156. doi:10.1128/MMBR.62.4.1094-1156.1998. PMC 98941. PMID 9841667.
- Kashyap S, Sarkar M (April 2010). "Mycoplasma pneumonia: Clinical features and management". Lung India. 27 (2): 75–85. doi:10.4103/0970-2113.63611. PMC 2893430. PMID 20616940.
- Narita M (September 2009). "Pathogenesis of neurologic manifestations of Mycoplasma pneumoniae infection". Pediatric Neurology. 41 (3): 159–66. doi:10.1016/j.pediatrneurol.2009.04.012. PMID 19664529.
- Ferwerda A, Moll HA, de Groot R (August 2001). "Respiratory tract infections by Mycoplasma pneumoniae in children: a review of diagnostic and therapeutic measures". European Journal of Pediatrics. 160 (8): 483–91. doi:10.1007/s004310100775. PMID 11548186. S2CID 9131256.
- Esposito S, Droghetti R, Bosis S, Claut L, Marchisio P, Principi N (August 2002). "Cytokine secretion in children with acute Mycoplasma pneumoniae infection and wheeze". Pediatric Pulmonology. 34 (2): 122–7. doi:10.1002/ppul.10139. PMID 12112778. S2CID 1386332.
- Ríos AM, Mejías A, Chávez-Bueno S, Fonseca-Aten M, Katz K, Hatfield J, et al. (August 2004). "Impact of cethromycin (ABT-773) therapy on microbiological, histologic, immunologic, and respiratory indices in a murine model of Mycoplasma pneumoniae lower respiratory infection". Antimicrobial Agents and Chemotherapy. 48 (8): 2897–904. doi:10.1128/AAC.48.8.2897-2904.2004. PMC 478543. PMID 15273098.
- See also Hayflick's comments on Meredith Wadman's book, "The Vaccine Race: Science, Politics and the Human Costs of Defeating Disease", 2017 Errors in "The Vaccine Race" book