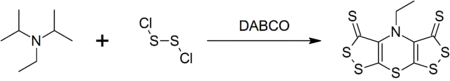| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
N-Ethyl-N-(propan-2-yl)propan-2-amine | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.027.629 | ||
| EC Number |
| ||
| MeSH | N,N-diisopropylethylamine | ||
PubChem CID |
|||
| UNII | |||
| UN number | 2733 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C8H19N | |||
| Molar mass | 129.247 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Fishy, ammoniacal | ||
| Density | 0.742 g mL−1 | ||
| Melting point | −50 to −46 °C (−58 to −51 °F; 223 to 227 K) | ||
| Boiling point | 126.6 °C; 259.8 °F; 399.7 K | ||
| 4.01 g/L (at 20 °C) | |||
| Vapor pressure | 4.1 kPa (at 37.70 °C) | ||
Refractive index (nD) |
1.414 | ||
| Hazards | |||
| GHS labelling: | |||
   | |||
| Danger | |||
| H225, H301, H314, H412 | |||
| P210, P273, P280, P301+P310, P305+P351+P338, P310 | |||
| Flash point | 10 °C (50 °F; 283 K) | ||
| Explosive limits | 0.7–6.3% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
200–500 mg kg−1 (oral, rat) | ||
| Related compounds | |||
Related amines |
|||
Related compounds |
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
N,N-Diisopropylethylamine, or Hünig's base, is an organic compound that is a tertiary amine. It is named after the German chemist Siegfried Hünig. It is used in organic chemistry as a non-nucleophilic base. It is commonly abbreviated as DIPEA, DIEA, or i-Pr2NEt.
Structure
DIPEA consists of a central nitrogen atom that is bonded to an ethyl group and two isopropyl groups. A lone pair of electrons resides on the nitrogen atom, which can react with electrophiles. However, the three alkyl groups on the nitrogen atom create steric hindrance, so only small electrophiles such as protons can react with the nitrogen lone pair.
Occurrence and preparation
DIPEA is commercially available. It is traditionally prepared by the alkylation of diisopropylamine with diethyl sulfate.[1]
Pure DIPEA exists as a colorless liquid, although commercial samples can be slightly yellow. If necessary, the compound can be purified by distillation from potassium hydroxide[2] or calcium hydride.[3]
Uses and reactions
DIPEA is a sterically hindered organic base that is commonly employed as a proton scavenger. Thus, like 2,2,6,6-tetramethylpiperidine and triethylamine, DIPEA is a good base but a poor nucleophile, DIPEA has low solubility in water, which makes it very easily recovered in commercial processes, a combination of properties that makes it a useful organic reagent.[4]
Amide coupling
It is commonly used as the hindered base in amide coupling reactions between a carboxylic acid (typically activated, for example, as an acid chloride, as illustrated below) and a nucleophilic amine.[5] As DIPEA is hindered and poorly nucleophilic, it does not compete with the nucleophilic amine in the coupling reaction.
Alkylations
DIPEA has been investigated for its use as a selective reagent in the alkylation of secondary amines to tertiary amines by alkyl halides. This is often hampered by an unwanted Menshutkin reaction forming a quaternary ammonium salt, but is absent when DIPEA is present.[6]
Transition metal catalyzed cross-coupling reactions
DIPEA can be used as a base in a number of transition metal catalyzed cross-coupling reactions, such as the Heck coupling and the Sonogashira coupling (as illustrated below).[7]
Swern oxidation
Although triethylamine is traditionally employed as the hindered base in Swern oxidations, the structurally similar DIPEA can be used instead, as exemplified below.[8]
Examples of DIPEA used as a substrate
DIPEA forms a complex heterocyclic compound called scorpionine (bis([1,2]dithiolo)-[1,4]thiazine) upon reaction with disulfur dichloride that is catalyzed by DABCO in a one-pot synthesis.[9]
Comparison with triethylamine
DIPEA and triethylamine are structurally very similar, with both compounds considered hindered organic bases. Due to their structural similarity, DIPEA and triethylamine can be used interchangeably in most applications. The nitrogen atom in DIPEA is more hindered than the nitrogen atom in triethylamine. However, triethylamine is a slightly stronger base than DIPEA; the pKa values of the respective conjugate acids in dimethyl sulfoxide are 9.0 and 8.5, respectively.[10]
References
- ↑ Hünig, S.; Kiessel, M. (1958). "Spezifische Protonenacceptoren als Hilfsbasen bei Alkylierungs- und Dehydrohalogenierungsreaktionen". Chemische Berichte. 91 (2): 380–392. doi:10.1002/cber.19580910223.
- ↑ Armarego, W. L. F. (2012-10-17). Purification of Laboratory Chemicals. Chai, Christina Li Lin (Seventh ed.). Amsterdam. ISBN 9780123821621. OCLC 820853648.
{{cite book}}: CS1 maint: location missing publisher (link) - ↑ Keiper, Sonja; Vyle, Joseph S. (2006-05-12). "Reversible Photocontrol of Deoxyribozyme-Catalyzed RNA Cleavage under Multiple-Turnover Conditions". Angewandte Chemie International Edition. 45 (20): 3306–3309. doi:10.1002/anie.200600164. ISSN 1433-7851. PMID 16619331. S2CID 21242501.
- ↑ Sorgi, K. L. (2001). "Diisopropylethylamine". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd254. ISBN 978-0471936237.
- ↑ Dunetz, Joshua R.; Magano, Javier; Weisenburger, Gerald A. (2016-02-05). "Large-Scale Applications of Amide Coupling Reagents for the Synthesis of Pharmaceuticals". Organic Process Research & Development. 20 (2): 140–177. doi:10.1021/op500305s. ISSN 1083-6160.
- ↑ Moore, J. L.; Taylor, S. M.; Soloshonok, V. A. (2005). "An efficient and operationally convenient general synthesis of tertiary amines by direct alkylation of secondary amines with alkyl halides in the presence of Huenig's base". Arkivoc. 2005 (part vi): 287–292. doi:10.3998/ark.5550190.0006.624. EJ-1549C.
- ↑ Chinchilla, Rafael; Nájera, Carmen (2011). "Recent advances in Sonogashira reactions". Chemical Society Reviews. 40 (10): 5084–5121. doi:10.1039/c1cs15071e. ISSN 0306-0012. PMID 21655588.
- ↑ Walba, David M.; Thurmes, William N.; Haltiwanger, R. Curtis (1988). "A highly stereocontrolled route to the monensin spiroketal ring system". The Journal of Organic Chemistry. 53 (5): 1046–1056. doi:10.1021/jo00240a022. ISSN 0022-3263.
- ↑ Rees, W.; Marcos, C. F.; Polo, C.; Torroba, T.; O. A. Rakitin (1997). "From Hünig's Base to Bis([1,2]dithiolo)-[1,4]thiazines in One Pot: The Fast Route to Highly Sulfurated Heterocycles". Angewandte Chemie International Edition. 36 (3): 281–283. doi:10.1002/anie.199702811.
- ↑ Lepore, Salvatore D.; Khoram, Anita; Bromfield, Deborah C.; Cohn, Pamela; Jairaj, Vinod; Silvestri, Maximilian A. (2005). "Studies on the Manganese-Mediated Isomerization of Alkynyl Carbonyls to Allenyl Carbonyls". The Journal of Organic Chemistry. 70 (18): 7443–7446. doi:10.1021/jo051040u. ISSN 0022-3263. PMID 16122274.