 | |
| Identifiers | |
|---|---|
| ChemSpider |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| Properties[1] | |
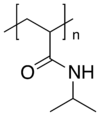
| (C6H11NO)n | |
| Molar mass | variable |
| Appearance | white solid |
| Density | 1.1 g/cm3 |
| Melting point | 96 °C (205 °F; 369 K) |
| Hazards[1] | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Poly(N-isopropylacrylamide) (variously abbreviated PNIPA, PNIPAM, PNIPAAm, NIPA, PNIPAA or PNIPAm) is a temperature-responsive polymer that was first synthesized in the 1950s.[2] It can be synthesized from N-isopropylacrylamide which is commercially available. It is synthesized via free-radical polymerization and is readily functionalized making it useful in a variety of applications.
PNIPA dissolves in water, however, when these solutions are heated in above their cloud point temperature, they undergo a reversible lower critical solution temperature (LCST) phase transition from a soluble hydrated state to an insoluble dehydrated state. Although it is widely believed that this phase transition occurs at 32 °C (90 °F),[3] the actual temperatures may differ 5 to 10 °C (or even more) depending on the polymer concentration,[3] molar mass of polymer chains, polymer dispersity as well as terminal moieties.[3][4] Furthermore, other molecules in the polymer solution, such as salts or proteins, can alter the cloud point temperature.[5][6]
Since PNIPA expels its liquid contents at a temperature near that of the human body, PNIPA copolymers have been investigated by many researchers for possible applications in tissue engineering[7][8] and controlled drug delivery.[9][10][11]
History
The synthesis of poly(N-isopropylacrylamide) began with the synthesis of the acrylamide monomer by Sprecht in 1956.[12] In 1957, Shearer patented the first application for what would be later identified as PNIPA for the use as a rodent repellent.[13] Early work was piqued by theoretical curiosity of the material properties of PNIPA. The first report of PNIPA came in 1968, which elucidated the unique thermal behavior in aqueous solutions.[14] The 1980s marked an explosion in interest in PNIPAs with the realization of potential applications due to its unique thermal behavior in aqueous solutions.[2]
Chemical and Physical Properties
PNIPA is one of the most studied thermosensitive hydrogels. In dilute solution, it undergoes a coil-to-globule transition.[15] PNIPA possesses an inverse solubility upon heating. It changes hydrophilicity and hydrophobicity abruptly at its LCST.[16] At lower temperatures PNIPA orders itself in solution in order to hydrogen bond with the already arranged water molecules. The water molecules must reorient around the nonpolar regions of PNIPA which results in a decreased entropy. At lower temperatures, such as room temperature, the negative enthalpy term () from hydrogen bonding effects dominates the Gibbs free energy,
causing the PNIPA to absorb water and dissolve in solution. At higher temperatures, the entropy term () dominates, causing the PNIPA to release water and phase separate which can be seen in the following demonstration.
 Aqueous solution of PNIPA before heating. The negative enthalpy of hydrogen bonding dominates and PNIPA is dissolved in the solution.
Aqueous solution of PNIPA before heating. The negative enthalpy of hydrogen bonding dominates and PNIPA is dissolved in the solution. The mixture after heating with a heat gun. The negative entropy of mixing dominates due to the increase in temperature and PNIPA phase separates from the water.
The mixture after heating with a heat gun. The negative entropy of mixing dominates due to the increase in temperature and PNIPA phase separates from the water.
Synthesis of Heat and pH Sensitive PNIPA
Homopolymerization[17]
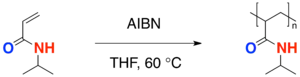
- The process of free radical polymerization of a single type of monomer, in this case, N-isopropylacrylamide, to form the polymer is known as a homopolymerization. The radical initiator azobisisobutyronitrile (AIBN) is commonly used in radical polymerizations.
Copolymerization
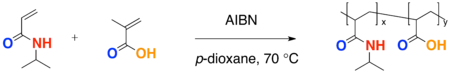
- A free-radical polymerization of two different monomer results in a copolymerization. An advantage to a copolymerization includes fine tuning of the LCST.
Terpolymerization
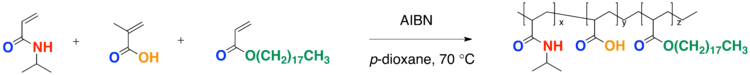
- A free-radical polymerization of three different monomer is known as a terpolymerization. Advantages to a terpolymerization may include enhancing multiple properties of the polymer including thermosensitivity, pH sensitivity or fine tuning of the LCST.
Cross-linked Hydrogel
- The reaction scheme below is a terpolymerization to form a cross-linked hydrogel. The reactant ammonium persulfate (APS) is used in polymer chemistry as a strong oxidizing agent that is often used along with tetramethylethylenediamine (TMEDA) to catalyze the polymerization when making polyacrylamide gels.

Synthesis of Chain-End Functionalized PNIPA
PNIPA can be functionalized using chain transfer agents using a free radical polymerization. The three schemes below demonstrate functionalization using chain transfer agents (CTA), where one end of the polymer is the radical initiator and the other is a functionalized group. Functionalization of the polymer chain-end allows for the polymer to be used in many diverse settings and applications. Advantages to a functionalizing the chain-end may include enhancing multiple properties of the polymer including thermosensitivity, pH sensitivity or fine tuning of the LCST.[17]
(1) 
(2) 
(3) 
Applications
The versatility of PNIPA has led to finding uses in macroscopic gels, microgels,[18] membranes, sensors, biosensors, thin films,[19][20][21] tissue engineering, and drug delivery. The tendency of aqueous solutions of PNIPA to increase in viscosity in the presence of hydrophobic molecules has made it excellent for tertiary oil recovery.
As aqueous solutions of PNIPA have their lower critical solution temperature in temperatures around human body temperatures, these polymers can be dissolved in water at room temperature and administered into body.[22] However, upon the administration, these polymers phase-separate and form insoluble aggregates at site of administration (this process is called thermogelling).[22] When PNIPA is administered into muscles of mice, its half-life was approximately 48 days (Mw = 20 kg/mol) and 66 days (Mw = 32 kg/mol) and caused no local or systemic pathologies.[22] Such phase separated hydrogels can be used for local drug delivery applications.[23] The PNIPA can be placed in a solution of bioactive molecules, which allows the bioactive molecules to penetrate the PNIPA. The PNIPA can then be placed in vivo, where there is a rapid release of biomolecules due to the initial gel collapse and an ejection of the biomolecules into the surrounding media, followed by a slow release of biomolecules due to surface pore closure.[24]
PNIPA have also been used in pH-sensitive drug delivery systems. Some examples of these drug delivery systems may include the intestinal delivery of human calcitonin,[25] delivery of insulin,[25] and the delivery of ibuprofen.[26] When radiolabeled PNIPA copolymers with different molecular weights were intravenously injected to rats, it was found that the glomerular filtration threshold of the polymer was around 32 000 g/mol.[27]
PNIPA have been used in gel actuators, which convert external stimuli into mechanical motion.[28] Upon heating above the LCST, the hydrogel goes from hydrophilic to hydrophobic state.[29] This conversion results in an expulsion of water which causes a physical conformational change, creating a mechanical hinge movement.
Furthermore, PNIPA-based thin films can be applied as nano-switches featuring multiple distinct thin-film states, which is based on the cononsolvency effect.[30][31][32]
References
- 1 2 "Poly(N-isopropylacrylamide) Material Safety Data Sheet". sigmaadlrich.com. Retrieved January 24, 2014.
- 1 2 Schild, H.G. (1992). "Poly(N-isopropylacrylamide): Experiment, theory and application". Progress in Polymer Science. 17 (2): 163–249. doi:10.1016/0079-6700(92)90023-R.
- 1 2 3 Halperin A, Kröger M, Winnik FM (2015). "Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research". Angew Chem Int Ed Engl. 54 (51): 15342–67. doi:10.1002/anie.201506663. PMID 26612195.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Aseyev, Vladimir; Tenhu, Heikki; Winnik, Françoise M. (2010). "Non-ionic Thermoresponsive Polymers in Water". Advances in Polymer Science. Vol. 242. Berlin, Heidelberg: Springer Berlin Heidelberg. pp. 29–89. doi:10.1007/12_2010_57. ISBN 978-3-642-22296-2. ISSN 0065-3195.
- ↑ Kolouchová, Kristýna; Lobaz, Volodymyr; Beneš, Hynek; et al. (2021). "Thermoresponsive properties of polyacrylamides in physiological solutions". Polymer Chemistry. Royal Society of Chemistry (RSC). 12 (35): 5077–5084. doi:10.1039/d1py00843a. hdl:1854/LU-8724379. ISSN 1759-9954. S2CID 238937814.
- ↑ Zhang, Yanjie; Furyk, Steven; Sagle, Laura B.; et al. (2007). "Effects of Hofmeister Anions on the LCST of PNIPAM as a Function of Molecular Weight†". The Journal of Physical Chemistry C. American Chemical Society (ACS). 111 (25): 8916–8924. doi:10.1021/jp0690603. ISSN 1932-7447. PMC 2553222. PMID 18820735.
- ↑ von Recum, H. A.; Kikuchi, A.; Okuhara, M.; Sakurai, Y.; Okano, T.; Kim, S. W. (1998). "Retinal pigmented epithelium cultures on thermally responsive polymer porous substrates". Journal of Biomaterials Science, Polymer Edition. 9 (11): 1241–1253. doi:10.1163/156856298X00758. PMID 9860183.
- ↑ Lee, EL.; von Recum, HA (2010). "Cell culture platform with mechanical conditioning and nondamaging cellular detachment". J Biomed Mater Res A. 93 (2): 411–8. doi:10.1002/jbm.a.32754. PMID 20358641. S2CID 23022529.
- ↑ Chung, J. E.; Yokoyama, M.; Yamato, M.; et al. (1999). "Thermo-responsive drug delivery from polymeric micelles constructed using block copolymers of poly(N-isopropylacrylamide) and poly(butylmethacrylate)". Journal of Controlled Release. 62 (1–2): 115–127. doi:10.1016/S0168-3659(99)00029-2. PMID 10518643.
- ↑ Yan, Hu; Tsujii, Kaoru (2005). "Potential application of poly(N-isopropylacrylamide) gel containing polymeric micelles to drug delivery systems". Colloids and Surfaces B: Biointerfaces. 46 (3): 142–146. doi:10.1016/j.colsurfb.2005.10.007. hdl:2115/1381. PMID 16300934. S2CID 31084515.
- ↑ Filipe E. Antunes; Luigi Gentile; Lorena Tavano; Cesare Oliviero Rossi (2009). "Rheological characterization of the thermal gelation of poly(N-isopropylacrylamide) and poly(N-isopropylacrylamide)co-Acrylic Acid". Applied Rheology. doi:10.3933/ApplRheol-19-42064.
- ↑ US 2773063
- ↑ US 2790744
- ↑ M. Heskins; J. E. Guillet (1968). "Solution Properties of Poly(N-isopropylacrylamide)". Journal of Macromolecular Science, Part A. 2 (8): 1441–1455. doi:10.1080/10601326808051910.
- ↑ Wu, C; Wang, X (1998). "Globule-to-Coil Transition of a Single Homopolymer Chain in Solution" (PDF). Physical Review Letters. 80 (18): 4092–4094. Bibcode:1998PhRvL..80.4092W. doi:10.1103/PhysRevLett.80.4092. Archived from the original (PDF) on July 21, 2011. Retrieved September 25, 2010.
- ↑ Somasundaran, Ponisseril (2004). Encyclopedia of Surface and Colloid Science. Taylor & Francis. ISBN 978-0-8247-2154-1. Retrieved February 13, 2014.
- 1 2 "Designing temperature and pH sensitive NIPAM based polymers". sigmaadlrich.com. Retrieved January 25, 2014.
- ↑ Widmann, Tobias; Kreuzer, Lucas P.; Hohn, Nuri; et al. (December 10, 2019). "Hydration and Solvent Exchange Induced Swelling and Deswelling of Homogeneous Poly(N-isopropylacrylamide) Microgel Thin Films". Langmuir. 35 (49): 16341–16352. doi:10.1021/acs.langmuir.9b03104. ISSN 0743-7463. PMID 31714092. S2CID 207940427.
- ↑ Kreuzer, Lucas P.; Widmann, Tobias; Hohn, Nuri; et al. (May 14, 2019). "Swelling and Exchange Behavior of Poly(sulfobetaine)-Based Block Copolymer Thin Films". Macromolecules. 52 (9): 3486–3498. Bibcode:2019MaMol..52.3486K. doi:10.1021/acs.macromol.9b00443. ISSN 0024-9297. S2CID 155174181.
- ↑ Kreuzer, Lucas P.; Widmann, Tobias; Bießmann, Lorenz; et al. (April 28, 2020). "Phase Transition Kinetics of Doubly Thermoresponsive Poly(sulfobetaine)-Based Diblock Copolymer Thin Films". Macromolecules. 53 (8): 2841–2855. Bibcode:2020MaMol..53.2841K. doi:10.1021/acs.macromol.0c00046. ISSN 0024-9297. S2CID 216346530.
- ↑ Kreuzer, Lucas P.; Widmann, Tobias; Aldosari, Nawarah; et al. (October 27, 2020). "Cyclic Water Storage Behavior of Doubly Thermoresponsive Poly(sulfobetaine)-Based Diblock Copolymer Thin Films". Macromolecules. 53 (20): 9108–9121. Bibcode:2020MaMol..53.9108K. doi:10.1021/acs.macromol.0c01335. ISSN 0024-9297. S2CID 226323489.
- 1 2 3 Groborz, Ondřej; Kolouchová, Kristýna; Pankrác, Jan; et al. (2022). "Pharmacokinetics of Intramuscularly Administered Thermoresponsive Polymers". Advanced Healthcare Materials. 11 (22). doi:10.1002/adhm.202201344. ISSN 2192-2640. PMID 36153823.
- ↑ A. T. Okano; Y. H. Bae; H. Jacobs; S. W. Kim (1990). "Thermally on-off switching polymers for drug permeation and release". Journal of Controlled Release. 11 (1–3): 255–265. doi:10.1016/0168-3659(90)90138-j.
- ↑ Allan S. Hoffman; Ali Afrassiabi; Liang Chang Dong (1986). "Thermally reversible hydrogels: II. Delivery and selective removal of substances from aqueous solutions". Journal of Controlled Release. 4 (3): 213–222. doi:10.1016/0168-3659(86)90005-2.
- 1 2 Schmaljohann, Dirk (2006). "Thermo- and pH-responsive polymers in drug delivery" (PDF). Advanced Drug Delivery Reviews. Elsevier. 58 (15): 1655–70. doi:10.1016/j.addr.2006.09.020. PMID 17125884. Retrieved March 13, 2014.
- ↑ Zhu, Senmin; Zhou, Zhengyang; Zhang, Di (March 15, 2007). "Grafting of thermo-responsive polymer inside mesoporous silica with large pore size using ATRP and investigation of its use in drug release". J. Mater. Chem. 17 (23): 2428–2433. doi:10.1039/b618834f.
- ↑ Bertrand, N.; Fleischer, J.G.; Wasan, K.M.; et al. (2009). "Pharmacokinetics and biodistribution of N-isopropylacrylamide copolymers for the design of pH-sensitive liposomes". Biomaterials. 30 (13): 2598–2605. doi:10.1016/j.biomaterials.2008.12.082. PMID 19176241.
- ↑ Chandorkar, Yashoda; Castro Nava, Arturo; Schweizerhof, Sjören; et al. (September 6, 2019). "Cellular responses to beating hydrogels to investigate mechanotransduction". Nature Communications. Springer Science and Business Media LLC. 10 (1): 4027. Bibcode:2019NatCo..10.4027C. doi:10.1038/s41467-019-11475-4. ISSN 2041-1723. PMC 6731269. PMID 31492837.
- ↑ Xiaobo Zhang; Cary L. Pint; Min Hyung Lee; et al. (2011). "Optically- and Thermally-Responsive Programmable Materials Based on Carbon Nanotube-Hydrogel Polymer Composites". Nano Letters. 11 (8): 3239–3244. Bibcode:2011NanoL..11.3239Z. doi:10.1021/nl201503e. PMID 21736337. S2CID 14317595.
- ↑ Kreuzer, Lucas P.; Geiger, Christina; Widmann, Tobias; et al. (August 10, 2021). "Solvation Behavior of Poly(sulfobetaine)-Based Diblock Copolymer Thin Films in Mixed Water/Methanol Vapors". Macromolecules. 54 (15): 7147–7159. Bibcode:2021MaMol..54.7147K. doi:10.1021/acs.macromol.1c01179. ISSN 0024-9297. S2CID 237724968.
- ↑ Kreuzer, Lucas P.; Lindenmeir, Christoph; Geiger, Christina; et al. (February 9, 2021). "Poly(sulfobetaine) versus Poly(N-isopropylmethacrylamide): Co-Nonsolvency-Type Behavior of Thin Films in a Water/Methanol Atmosphere". Macromolecules. 54 (3): 1548–1556. Bibcode:2021MaMol..54.1548K. doi:10.1021/acs.macromol.0c02281. ISSN 0024-9297. S2CID 234184714.
- ↑ Geiger, Christina; Reitenbach, Julija; Henschel, Cristiane; et al. (November 2021). "Ternary Nanoswitches Realized with Multiresponsive PMMA‐ b ‐PNIPMAM Films in Mixed Water/Acetone Vapor Atmospheres". Advanced Engineering Materials. 23 (11): 2100191. doi:10.1002/adem.202100191. ISSN 1438-1656. S2CID 235560292.
