 | |
| Clinical data | |
|---|---|
| Trade names | Netromycin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605011 |
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~0% |
| Elimination half-life | 2.5 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.054.661 |
| Chemical and physical data | |
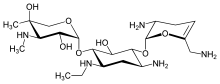
| Formula | C21H41N5O7 |
| Molar mass | 475.587 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Netilmicin (1-N-ethylsisomicin) is a semisynthetic aminoglycoside antibiotic, and a derivative of sisomicin, produced by Micromonospora inyoensis. Aminoglycoside antibiotics have the ability to kill a wide variety of bacteria. Netilmicin is not absorbed from the gut and is therefore only given by injection or infusion. It is only used in the treatment of serious infections particularly those resistant to gentamicin.
It was patented in 1973 and approved for medical use in 1981.[1] It was approved for medical use in the UK in December 2019, for the treatment of external infections of the eye.[2] It is on the World Health Organization's List of Essential Medicines.[3]
Comparison with drugs
According to the British National Formulary (BNF), netilmicin has similar activity to gentamicin, but less ototoxicity in those needing treatment for longer than 10 days. Netilmicin is active against a number of gentamicin-resistant Gram-negative bacteria but is less active against Pseudomonas aeruginosa than gentamicin or tobramycin.
References
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 508. ISBN 9783527607495.
- ↑ "Netilmicin". SPS - Specialist Pharmacy Service. 2 June 2020. Retrieved 21 August 2020.
- ↑ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
Further reading
- Wright J (1976). "Synthesis of 1-N-ethylsisomicin: a broad-spectrum semisynthetic aminoglycoside antibiotic". Journal of the Chemical Society, Chemical Communications (6): 206–208. doi:10.1039/C39760000206.
- Hemsworth S, Nunn A, Selwood K, Osborne C, Jones A, Pizer B (2005). "Once-daily netilmicin for neutropenic pyrexia in paediatric oncology". Acta Paediatr. 94 (3): 268–74. doi:10.1080/08035250510025923. PMID 16028643.
- Klingenberg C, Småbrekke L, Lier T, Flaegstad T (2004). "Validation of a simplified netilmicin dosage regimen in infants". Scand J Infect Dis. 36 (6–7): 474–9. doi:10.1080/00365540410020613. PMID 15307571. S2CID 29092705.
- Brooks J, Marlow N, Reeves B, Millar M (2004). "Use of once-daily netilmicin to treat infants with suspected sepsis in a neonatal intensive care unit". Biol Neonate. 86 (3): 170–5. doi:10.1159/000079423. PMID 15237240. S2CID 37410607.
External links
- "Netilmicin". Drug Information Portal. U.S. National Library of Medicine.