| |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(1s,4s)-1-(2-Carboxy-2-oxoethyl)-4-hydroxycyclohexa-2,5-diene-1-carboxylic acid | |
| Other names
Prephenate; cis-1-Carboxy-4-hydroxy-α-oxo-2,5-cyclohexadiene-1-propanoic acid | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| MeSH | Prephenic+acid |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H10O6 | |
| Molar mass | 226.184 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Prephenic acid, commonly also known by its anionic form prephenate, is an intermediate in the biosynthesis of the aromatic amino acids phenylalanine and tyrosine, as well as of a large number of secondary metabolites of the shikimate pathway.
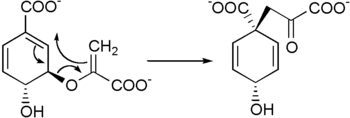
It is biosynthesized by a [3,3]-sigmatropic Claisen rearrangement of chorismate.[1][2]
Stereochemistry
Prephenic acid is an example of achiral (optically inactive) molecule which has two pseudoasymmetric atoms (i.e. stereogenic but not chirotopic centers), the C1 and the C4 cyclohexadiene ring atoms. It has been shown[3] that of the two possible diastereoisomers, the natural prephenic acid is one that has both substituents at higher priority (according to CIP rules) on the two pseudoasymmetric carbons, i.e. the carboxyl and the hydroxyl groups, in the cis configuration, or (1s,4s) according to the new IUPAC stereochemistry rules (2013).[4]
The other stereoisomer, i.e. trans or, better, (1r,4r), is called epiprephenic.
See also
References
- ↑ Helmut Goerisch (1978). "On the mechanism of the chorismate mutase reaction". Biochemistry. 17 (18): 3700–3705. doi:10.1021/bi00611a004. PMID 100134.
- ↑ Peter Kast, Yadu B. Tewari, Olaf Wiest, Donald Hilvert, Kendall N. Houk, and Robert N. Goldberg (1997). "Thermodynamics of the Conversion of Chorismate to Prephenate: Experimental Results and Theoretical Predictions". J. Phys. Chem. B. 101 (50): 10976–10982. doi:10.1021/jp972501l.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Danishefsky, Samuel; Hirama, Masahiro; Fritsch, Nancy; Clardy, Jon (1979-11-01). "Synthesis of disodium prephenate and disodium epiprephenate. Stereochemistry of prephenic acid and an observation on the base-catalyzed rearrangement of prephenic acid to p-hydroxyphenyllactic acid". Journal of the American Chemical Society. 101 (23): 7013–7018. doi:10.1021/ja00517a039. ISSN 0002-7863.
- ↑ Favre, Henri A; Powell, Warren H (2013-12-17). Nomenclature of Organic Chemistry. doi:10.1039/9781849733069. ISBN 9780854041824.