| Identifiers | |
|---|---|
3D model (JSmol) |
|
| EC Number |
|
PubChem CID |
|
| |
| |
| Properties | |
| H2Na6O40W12 | |
| Molar mass | 2985.99 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
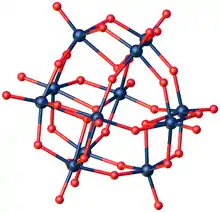
Sodium metatungstate is the inorganic compound with the formula Na6[H2W12O40], sometimes written 3Na2WO4·9WO3·H2O. It is also referred to as sodium polytungstate (SPT). This salt has been used in the manufacture of dense aqueous solutions. Sodium metatungstate exists as white solid. The anion is the polyoxotungstate [H2W12O40]6-, which features six-coordinated tungsten(VI) centers interconnected with doubly- and triply bridging oxo ligands.
Due to its very high solubility in water (max. density 3.1 g/cm3), SPT is widely used as to produce "heavy liquid" for gravity separation (sink /float analysis) and density gradient centrifugation. It has significant advantages when compared to zinc chloride solution or the toxic halogenated carbons for sink-swim analysis. Aqueous SPT is non-toxic (unlike the denser Clerici solution), non-flammable, odorless, reusable and additionally it has a low viscosity.[2]
References
- ↑ Asami, M.; Ichida, H.; Sasaki, Y. (1984). "The structure of hexakis(tetramethylammonium) dihydrogendodecatungstate enneahydrate, [(CH3)4N]6[H2W12O40].9H2O". Acta Crystallographica Section C. 40: 35–37. doi:10.1107/S0108270184002924.
- ↑ Munsterman, Dirk; Kerstholt, Susan (1996). "Sodium polytungstate, a new non-toxic alternative to bromoform in heavy liquid separation". Review of Palaeobotany and Palynology. 91 (1–4): 417–422. Bibcode:1996RPaPa..91..417M. doi:10.1016/0034-6667(95)00093-3.
Further reading
- Gregory, Murray R.; Johnston, Keith A. (1987). "A nontoxic substitute for hazardous heavy liquids—aqueous sodium polytungstate (3Na2WO4.9WO3.H2O) solution (Note)". New Zealand Journal of Geology and Geophysics. 30 (3): 317. doi:10.1080/00288306.1987.10552626.
- Bolch, C. J. S. (1997). "The use of sodium polytungstate for the separation and concentration of living dinoflagellate cysts from marine sediments". Phycologia. 36 (6): 472–478. doi:10.2216/i0031-8884-36-6-472.1.
- Six, J (1999). "Recycling of sodium polytungstate used in soil organic matter studies". Soil Biology and Biochemistry. 31 (8): 1193–1196. doi:10.1016/S0038-0717(99)00023-1.
- Savage, N. M. (1988). "The use of sodium polytungstate for conodont separations" (PDF). Journal of Micropalaeontology. 7 (1): 39–40. Bibcode:1988JMicP...7...39S. doi:10.1144/jm.7.1.39. S2CID 129701930.