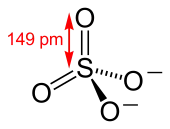 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Sulfate | |||
| Other names
Tetraoxosulfate(VI) Tetraoxidosulfate(VI) | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.108.048 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| SO2−4 | |||
| Molar mass | 96.06 g·mol−1 | ||
| Conjugate acid | Hydrogensulfate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
The sulfate or sulphate ion is a polyatomic anion with the empirical formula SO2−4. Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid.
Spelling
"Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English.
Structure
The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry of the isolated anion is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge of −2 and it is the conjugate base of the bisulfate (or hydrogensulfate) ion, HSO−4, which is in turn the conjugate base of H2SO4, sulfuric acid. Organic sulfate esters, such as dimethyl sulfate, are covalent compounds and esters of sulfuric acid. The tetrahedral molecular geometry of the sulfate ion is as predicted by VSEPR theory.
Bonding
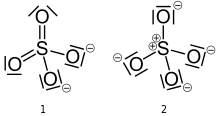

The first description of the bonding in modern terms was by Gilbert Lewis in his groundbreaking paper of 1916 where he described the bonding in terms of electron octets around each atom, that is no double bonds and a formal charge of +2 on the sulfur atom and -1 on each oxygen atom.[1][lower-alpha 1]
Later, Linus Pauling used valence bond theory to propose that the most significant resonance canonicals had two pi bonds involving d orbitals. His reasoning was that the charge on sulfur was thus reduced, in accordance with his principle of electroneutrality.[2] The S−O bond length of 149 pm is shorter than the bond lengths in sulfuric acid of 157 pm for S−OH. The double bonding was taken by Pauling to account for the shortness of the S−O bond. Pauling's use of d orbitals provoked a debate on the relative importance of pi bonding and bond polarity (electrostatic attraction) in causing the shortening of the S−O bond. The outcome was a broad consensus that d orbitals play a role, but are not as significant as Pauling had believed.[3][4]
A widely accepted description involving pπ – dπ bonding was initially proposed by Durward William John Cruickshank. In this model, fully occupied p orbitals on oxygen overlap with empty sulfur d orbitals (principally the dz2 and dx2–y2).[5] However, in this description, despite there being some π character to the S−O bonds, the bond has significant ionic character. For sulfuric acid, computational analysis (with natural bond orbitals) confirms a clear positive charge on sulfur (theoretically +2.45) and a low 3d occupancy. Therefore, the representation with four single bonds is the optimal Lewis structure rather than the one with two double bonds (thus the Lewis model, not the Pauling model).[6] In this model, the structure obeys the octet rule and the charge distribution is in agreement with the electronegativity of the atoms. The discrepancy between the S−O bond length in the sulfate ion and the S−OH bond length in sulfuric acid is explained by donation of p-orbital electrons from the terminal S=O bonds in sulfuric acid into the antibonding S−OH orbitals, weakening them resulting in the longer bond length of the latter.
However, the bonding representation of Pauling for sulfate and other main group compounds with oxygen is still a common way of representing the bonding in many textbooks.[5][7] The apparent contradiction can be cleared if one realizes that the covalent double bonds in the Lewis structure in reality represent bonds that are strongly polarized by more than 90% towards the oxygen atom. On the other hand, in the structure with a dipolar bond, the charge is localized as a lone pair on the oxygen.[6]
Preparation
Typically metal sulfates are prepared by treating metal oxides, metal carbonates, or the metal itself with sulfuric acid:[7]
- Zn + H2SO4 → ZnSO4 + H2
- Cu(OH)2 + H2SO4 → CuSO4 + 2 H2O
- CdCO3 + H2SO4 → CdSO4 + H2O + CO2
Although written with simple anhydrous formulas, these conversions generally are conducted in the presence of water. Consequently the product sulfates are hydrated, corresponding to zinc sulfate ZnSO4·7H2O, copper(II) sulfate CuSO4·5H2O, and cadmium sulfate CdSO4·H2O.
Some metal sulfides can be oxidized to give metal sulfates.
Properties
There are numerous examples of ionic sulfates, many of which are highly soluble in water. Exceptions include calcium sulfate, strontium sulfate, lead(II) sulfate, barium sulfate, silver sulfate, and mercury sulfate, which are poorly soluble. Radium sulfate is the most insoluble sulfate known. The barium derivative is useful in the gravimetric analysis of sulfate: if one adds a solution of most barium salts, for instance barium chloride, to a solution containing sulfate ions, barium sulfate will precipitate out of solution as a whitish powder. This is a common laboratory test to determine if sulfate anions are present.
The sulfate ion can act as a ligand attaching either by one oxygen (monodentate) or by two oxygens as either a chelate or a bridge.[7] An example is the complex Co(en)2(SO4)]+Br−[7] or the neutral metal complex PtSO4(PPh3)2] where the sulfate ion is acting as a bidentate ligand. The metal–oxygen bonds in sulfate complexes can have significant covalent character.
Uses and occurrence
Commercial applications
.jpg.webp)
Sulfates are widely used industrially. Major compounds include:
- Gypsum, the natural mineral form of hydrated calcium sulfate, is used to produce plaster. About 100 million tonnes per year are used by the construction industry.
- Copper sulfate, a common algaecide, the more stable form (CuSO4) is used for galvanic cells as electrolyte
- Iron(II) sulfate, a common form of iron in mineral supplements for humans, animals, and soil for plants
- Magnesium sulfate (commonly known as Epsom salts), used in therapeutic baths
- Lead(II) sulfate, produced on both plates during the discharge of a lead–acid battery
- Sodium laureth sulfate, or SLES, a common detergent in shampoo formulations
- Polyhalite, K2Ca2Mg(SO4)4·2H2O, used as fertiliser.
Occurrence in nature
Sulfate-reducing bacteria, some anaerobic microorganisms, such as those living in sediment or near deep sea thermal vents, use the reduction of sulfates coupled with the oxidation of organic compounds or hydrogen as an energy source for chemosynthesis.
History
Some sulfates were known to alchemists. The vitriol salts, from the Latin vitreolum, glassy, were so-called because they were some of the first transparent crystals known.[8] Green vitriol is iron(II) sulfate heptahydrate, FeSO4·7H2O; blue vitriol is copper(II) sulfate pentahydrate, CuSO4·5H2O and white vitriol is zinc sulfate heptahydrate, ZnSO4·7H2O. Alum, a double sulfate of potassium and aluminium with the formula K2Al2(SO4)4·24H2O, figured in the development of the chemical industry.
Environmental effects
Sulfates occur as microscopic particles (aerosols) resulting from fossil fuel and biomass combustion. They increase the acidity of the atmosphere and form acid rain. The anaerobic sulfate-reducing bacteria Desulfovibrio desulfuricans and D. vulgaris can remove the black sulfate crust that often tarnishes buildings.[9]
Main effects on climate



Reversal and accelerated warming

After 1990, the global dimming trend had clearly switched to global brightening.[15][16][17][18][13] This followed measures taken to combat air pollution by the developed nations, typically through flue-gas desulfurization installations at thermal power plants, such as wet scrubbers or fluidized bed combustion.[19][20] In the United States, sulfate aerosols have declined significantly since 1970 with the passage of the Clean Air Act, which was strengthened in 1977 and 1990. According to the EPA, from 1970 to 2005, total emissions of the six principal air pollutants, including sulfates, dropped by 53% in the US.[21] By 2010, this reduction in sulfate pollution led to estimated healthcare cost savings valued at $50 billion annually.[22] Similar measures were taken in Europe,[21] such as the 1985 Helsinki Protocol on the Reduction of Sulfur Emissions under the Convention on Long-Range Transboundary Air Pollution, and with similar improvements.[23]

Since changes in aerosol concentrations already have an impact on the global climate, they would necessarily influence future projections as well. In fact, it is impossible to fully estimate the warming impact of all greenhouse gases without accounting for the counteracting cooling from aerosols. Climate models started to account for the effects of sulfate aerosols around the IPCC Second Assessment Report; when the IPCC Fourth Assessment Report was published in 2007, every climate model had integrated sulfates, but only 5 were able to account for less impactful particulates like black carbon.[28] By 2021, CMIP6 models estimated total aerosol cooling in the range from 0.1 °C (0.18 °F) to 0.7 °C (1.3 °F);[29] The IPCC Sixth Assessment Report selected the best estimate of a 0.5 °C (0.90 °F) cooling provided by sulfate aerosols, while black carbon amounts to about 0.1 °C (0.18 °F) of warming.[30] While these values are based on combining model estimates with observational constraints, including those on ocean heat content,[31] the matter is not yet fully settled. The difference between model estimates mainly stems from disagreements over the indirect effects of aerosols on clouds.[32][33]
It has also been suggested that aerosols are not given sufficient attention in regional risk assessments, in spite of being more influential on a regional scale than globally.[34] For instance, a climate change scenario with high greenhouse gas emissions but strong reductions in air pollution would see 0.2 °C (0.36 °F) more global warming by 2050 than the same scenario with little improvement in air quality, but regionally, the difference would add 5 more tropical nights per year in northern China and substantially increase precipitation in northern China and northern India.[35] Likewise, a paper comparing current level of clean air policies with a hypothetical maximum technically feasible action under otherwise the same climate change scenario found that the latter would increase the risk of temperature extremes by 30–50% in China and in Europe.[36] Unfortunately, because historical records of aerosols are sparser in some regions than in others, accurate regional projections of aerosol impacts are difficult. Even the latest CMIP6 climate models can only accurately represent aerosol trends over Europe,[10] but struggle with representing North America and Asia, meaning that their near-future projections of regional impacts are likely to contain errors as well.[37][10][38]Hydrological cycle

Solar geoengineering
Hydrogensulfate (bisulfate)
 | |
| Names | |
|---|---|
| IUPAC name
Hydrogensulfate[57] | |
| Other names
Bisulfate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.108.048 |
| 2121 | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| HSO−4 | |
| Molar mass | 97.071 g/mol |
| Conjugate acid | Sulfuric acid |
| Conjugate base | Sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The hydrogensulfate ion (HSO−4), also called the bisulfate ion, is the conjugate base of sulfuric acid (H2SO4).[58][lower-alpha 2] Sulfuric acid is classified as a strong acid; in aqueous solutions it ionizes completely to form hydronium (H3O+) and hydrogensulfate (HSO−4) ions. In other words, the sulfuric acid behaves as a Brønsted–Lowry acid and is deprotonated to form hydrogensulfate ion. Hydrogensulfate has a valency of 1. An example of a salt containing the HSO−4 ion is sodium bisulfate, NaHSO4. In dilute solutions the hydrogensulfate ions also dissociate, forming more hydronium ions and sulfate ions (SO2−4).
Other sulfur oxyanions
| Molecular formula | Name |
|---|---|
| SO2−5 | Peroxomonosulfate |
| SO2−4 | Sulfate |
| SO2−3 | Sulfite |
| S2O2−8 | Peroxydisulfate |
| S2O2−7 | Pyrosulfate |
| S2O2−6 | Dithionate |
| S2O2−5 | Metabisulfite |
| S2O2−4 | Dithionite |
| S2O2−3 | Thiosulfate |
| S3O2−6 | Trithionate |
| S4O2−6 | Tetrathionate |
See also
Notes
- ↑ Lewis assigned to sulfur a negative charge of two, starting from six own valence electrons and ending up with eight electrons shared with the oxygen atoms. In fact, sulfur donates two electrons to the oxygen atoms.
- ↑ The prefix "bi" in "bisulfate" comes from an outdated naming system and is based on the observation that there is twice as much sulfate (SO2−4) in sodium bisulfate (NaHSO4) and other bisulfates as in sodium sulfate (Na2SO4) and other sulfates. See also bicarbonate.
References
- ↑ Lewis, Gilbert N. (1916). "The Atom and the Molecule". J. Am. Chem. Soc. 38 (4): 762–785. doi:10.1021/ja02261a002. S2CID 95865413. (See page 778.)
- ↑ Pauling, Linus (1948). "The modern theory of valency". J. Chem. Soc. 17: 1461–1467. doi:10.1039/JR9480001461. PMID 18893624.
- ↑ Coulson, C. A. (1969). "d Electrons and Molecular Bonding". Nature. 221 (5186): 1106. Bibcode:1969Natur.221.1106C. doi:10.1038/2211106a0. S2CID 4162835.
- ↑ Mitchell, K. A. R. (1969). "Use of outer d orbitals in bonding". Chem. Rev. 69 (2): 157. doi:10.1021/cr60258a001.
- 1 2 Cotton, F. Albert; Wilkinson, Geoffrey (1966). Advanced Inorganic Chemistry (2nd ed.). New York, NY: Wiley.
- 1 2 Stefan, Thorsten; Janoschek, Rudolf (Feb 2000). "How relevant are S=O and P=O Double Bonds for the Description of the Acid Molecules H2SO3, H2SO4, and H3PO4, respectively?". J. Mol. Modeling. 6 (2): 282–288. doi:10.1007/PL00010730. S2CID 96291857.
- 1 2 3 4 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Taylor, F. Sherwood (1942). Inorganic and Theoretical Chemistry (6th ed.). William Heinemann.
- ↑ Andrea Rinaldi (Nov 2006). "Saving a fragile legacy. Biotechnology and microbiology are increasingly used to preserve and restore the worlds cultural heritage". EMBO Reports. 7 (11): 1075–1079. doi:10.1038/sj.embor.7400844. PMC 1679785. PMID 17077862.
- 1 2 3 Julsrud, I. R.; Storelvmo, T.; Schulz, M.; Moseid, K. O.; Wild, M. (20 October 2022). "Disentangling Aerosol and Cloud Effects on Dimming and Brightening in Observations and CMIP6". Journal of Geophysical Research: Atmospheres. 127 (21): e2021JD035476. doi:10.1029/2021JD035476.
- ↑ J. Srinivasan; et al. (2002). "Asian Brown Cloud – fact and fantasy" (PDF). Current Science. 83 (5): 586–592.
- ↑ Cohen, Shabtai; Stanhill, Gerald (1 January 2021), Letcher, Trevor M. (ed.), "Chapter 32 – Changes in the Sun's radiation: the role of widespread surface solar radiation trends in climate change: dimming and brightening", Climate Change (Third Edition), Elsevier, pp. 687–709, doi:10.1016/b978-0-12-821575-3.00032-3, ISBN 978-0-12-821575-3, S2CID 234180702, retrieved 2023-04-26
- 1 2 "Global 'Sunscreen' Has Likely Thinned, Report NASA Scientists". NASA. 15 March 2007.
- ↑ IPCC, 1990: Chapter 1: Greenhouse Gases and Aerosols [R.T. Watson, H. Rodhe, H. Oeschger and U. Siegenthaler]. In: Climate Change: The IPCC Scientific Assessment [J.T.Houghton, G.J.Jenkins and J.J.Ephraums (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 31–34,
- ↑ "Earth lightens up". Pacific Northwest National Laboratory. Retrieved 8 May 2005.
- ↑ Wild, M; et al. (2005). "From Dimming to Brightening: Decadal Changes in Solar Radiation at Earth's Surface". Science. 308 (2005–05–06): 847–850. Bibcode:2005Sci...308..847W. doi:10.1126/science.1103215. PMID 15879214. S2CID 13124021.
- ↑ Pinker; Zhang, B; Dutton, EG; et al. (2005). "Do Satellites Detect Trends in Surface Solar Radiation?". Science. 308 (6 May 2005): 850–854. Bibcode:2005Sci...308..850P. doi:10.1126/science.1103159. PMID 15879215. S2CID 10644227.
- ↑ "Global Dimming may have a brighter future". RealClimate. 15 May 2005. Retrieved 2006-06-12.
- ↑ Lin, Cheng-Kuan; Lin, Ro-Ting; Chen, Pi-Cheng; Wang, Pu; De Marcellis-Warin, Nathalie; Zigler, Corwin; Christiani, David C. (8 February 2018). "A Global Perspective on Sulfur Oxide Controls in Coal-Fired Power Plants and Cardiovascular Disease". Scientific Reports. 8 (1): 2611. Bibcode:2018NatSR...8.2611L. doi:10.1038/s41598-018-20404-2. ISSN 2045-2322. PMC 5805744. PMID 29422539.
- ↑ Lindeburg, Michael R. (2006). Mechanical Engineering Reference Manual for the PE Exam. Belmont, C.A.: Professional Publications, Inc. pp. 27–3. ISBN 978-1-59126-049-3.
- 1 2 3 "Air Emissions Trends – Continued Progress Through 2005". U.S. Environmental Protection Agency. 8 July 2014. Archived from the original on 2007-03-17. Retrieved 2007-03-17.
- 1 2 "Effects of Acid Rain – Human Health". EPA. 2 June 2006. Archived from the original on 18 January 2008. Retrieved 2 September 2013.
- ↑ Moses, Elizabeth; Cardenas, Beatriz; Seddon, Jessica (25 February 2020). "The Most Successful Air Pollution Treaty You've Never Heard Of".
- ↑ "Crichton's Thriller State of Fear: Separating Fact from Fiction". Archived from the original on 2006-06-14. Retrieved 12 June 2006.
- ↑ ""Warming Hole" Over the Eastern U.S. Due to Air Pollution". NASA. 18 May 2012.
- ↑ Karmalkar, Ambarish V.; Horton, Radley M. (23 September 2021). "Drivers of exceptional coastal warming in the northeastern United States". Nature Climate Change. 11 (10): 854–860. doi:10.1038/s41558-021-01159-7. S2CID 237611075.
- ↑ Krajick, Kevin (23 September 2021). "Why the U.S. Northeast Coast Is a Global Warming Hot Spot". Columbia Climate School. Retrieved 2023-03-23.
- ↑ "Aerosols and Incoming Sunlight (Direct Effects)". NASA. 2 November 2010.
- ↑ Gillett, Nathan P.; Kirchmeier-Young, Megan; Ribes, Aurélien; Shiogama, Hideo; Hegerl, Gabriele C.; Knutti, Reto; Gastineau, Guillaume; John, Jasmin G.; Li, Lijuan; Nazarenko, Larissa; Rosenbloom, Nan; Seland, Øyvind; Wu, Tongwen; Yukimoto, Seiji; Ziehn, Tilo (18 January 2021). "Constraining human contributions to observed warming since the pre-industrial period" (PDF). Nature Climate Change. 11 (3): 207–212. doi:10.1038/s41558-020-00965-9. S2CID 231670652.
- ↑ IPCC, 2021: Summary for Policymakers. In: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 3–32, doi:10.1017/9781009157896.001.
- ↑ Quaas, Johannes; Jia, Hailing; Smith, Chris; Albright, Anna Lea; Aas, Wenche; Bellouin, Nicolas; Boucher, Olivier; Doutriaux-Boucher, Marie; Forster, Piers M.; Grosvenor, Daniel; Jenkins, Stuart; Klimont, Zbigniew; Loeb, Norman G.; Ma, Xiaoyan; Naik, Vaishali; Paulot, Fabien; Stier, Philip; Wild, Martin; Myhre, Gunnar; Schulz, Michael (21 September 2022). "Robust evidence for reversal of the trend in aerosol effective climate forcing". Atmospheric Chemistry and Physics. 22 (18): 12221–12239. doi:10.5194/acp-22-12221-2022. hdl:20.500.11850/572791. S2CID 252446168.
- ↑ Andrew, Tawana (27 September 2019). "Behind the Forecast: How clouds affect temperatures". Science Behind the Forecast. LOUISVILLE, Ky. (WAVE). Retrieved 4 January 2023.
- ↑ Zhang, Jie; Furtado, Kalli; Turnock, Steven T.; Mulcahy, Jane P.; Wilcox, Laura J.; Booth, Ben B.; Sexton, David; Wu, Tongwen; Zhang, Fang; Liu, Qianxia (22 December 2021). "The role of anthropogenic aerosols in the anomalous cooling from 1960 to 1990 in the CMIP6 Earth system models". Atmospheric Chemistry and Physics. 21 (4): 18609–18627. doi:10.5194/acp-21-18609-2021.
- ↑ Persad, Geeta G.; Samset, Bjørn H.; Wilcox, Laura J. (21 November 2022). "Aerosols must be included in climate risk assessments". Nature. 611 (7937): 662–664. doi:10.1038/d41586-022-03763-9. PMID 36411334.
- ↑ Li, Yingfang; Wang, Zhili; Lei, Yadong; Che, Huizheng; Zhang, Xiaoye (23 February 2023). "Impacts of reductions in non-methane short-lived climate forcers on future climate extremes and the resulting population exposure risks in eastern and southern Asia". Atmospheric Chemistry and Physics. 23 (4): 2499–2523. doi:10.5194/acp-23-2499-2023. S2CID 257180147.
- ↑ Luo, Feifei; Wilcox, Laura; Dong, Buwen; Su, Qin; Chen, Wei; Dunstone, Nick; Li, Shuanglin; Gao, Yongqi (19 February 2020). "Projected near-term changes of temperature extremes in Europe and China under different aerosol emissions". Environmental Research Letters. 15 (3): 4013. doi:10.1088/1748-9326/ab6b34.
- ↑ Wang, Zhili; Lin, Lei; Xu, Yangyang; Che, Huizheng; Zhang, Xiaoye; Zhang, Hua; Dong, Wenjie; Wang, Chense; Gui, Ke; Xie, Bing (12 January 2021). "Incorrect Asian aerosols affecting the attribution and projection of regional climate change in CMIP6 models". npj Climate and Atmospheric Science. 4. doi:10.1029/2021JD035476.
- ↑ Ramachandran, S.; Rupakheti, Maheswar; Cherian, R. (10 February 2022). "Insights into recent aerosol trends over Asia from observations and CMIP6 simulations". Science of the Total Environment. 807 (1): 150756. doi:10.1016/j.scitotenv.2021.150756. PMID 34619211. S2CID 238474883.
- ↑ Xie, Xiaoning; Myhre, Gunnar; Shindell, Drew; Faluvegi, Gregory; Takemura, Toshihiko; Voulgarakis, Apostolos; Shi, Zhengguo; Li, Xinzhou; Xie, Xiaoxun; Liu, Heng; Liu, Xiaodong; Liu, Yangang (27 December 2022). "Anthropogenic sulfate aerosol pollution in South and East Asia induces increased summer precipitation over arid Central Asia". Communications Earth & Environment. 3 (1): 328. doi:10.1038/s43247-022-00660-x. PMC 9792934. PMID 36588543.
- ↑ Pan, Bowen; Wang, Yuan; Hu, Jiaxi; Lin, Yun; Hsieh, Jen-Shan; Logan, Timothy; Feng, Xidan; Jiang, Jonathan H.; Yung, Yuk L.; Zhang, Renyi (2018). "Sahara dust may make you cough, but it's a storm killer". Journal of Climate. 31 (18): 7621–7644. doi:10.1175/JCLI-D-16-0776.1.
- ↑ Cat Lazaroff (7 December 2001). "Aerosol Pollution Could Drain Earth's Water Cycle". Environment News Service. Archived from the original on 2016-06-03. Retrieved 2007-03-24.
- ↑ Kostel, Ken; Oh, Clare (14 April 2006). "Could Reducing Global Dimming Mean a Hotter, Dryer World?". Lamont–Doherty Earth Observatory News. Archived from the original on 2016-03-03. Retrieved 2006-06-12.
- ↑ Chang, C.-Y.; Chiang, J. C. H.; Wehner, M. F.; Friedman, A. R.; Ruedy, R. (15 May 2011). "Sulfate Aerosol Control of Tropical Atlantic Climate over the Twentieth Century". Journal of Climate. 24 (10): 2540–2555. doi:10.1175/2010JCLI4065.1.
- ↑ Peace, Amy H.; Booth, Ben B. B.; Regayre, Leighton A.; Carslaw, Ken S.; Sexton, David M. H.; Bonfils, Céline J. W.; Rostron, John W. (26 August 2022). "Evaluating uncertainty in aerosol forcing of tropical precipitation shifts". Earth System Dynamics. 13 (3): 1215–1232. doi:10.5194/esd-13-1215-2022.
- ↑ Allen, Robert J. (20 August 2015). "A 21st century northward tropical precipitation shift caused by future anthropogenic aerosol reductions". Journal of Geophysical Research: Atmospheres. 120 (18): 9087–9102. doi:10.1002/2015JD023623.
- ↑ Kobayashi, Yuya; Ide, Yu; Takegawa, Nobuyuki (2021-04-03). "Development of a novel particle mass spectrometer for online measurements of refractory sulfate aerosols". Aerosol Science and Technology. 55 (4): 371–386. Bibcode:2021AerST..55..371K. doi:10.1080/02786826.2020.1852168. ISSN 0278-6826. S2CID 229506768.
- ↑ Palumbo, P., A. Rotundi, V. Della Corte, A. Ciucci, L. Colangeli, F. Esposito, E. Mazzotta Epifani, V. Mennella , J.R. Brucato, F.J.M. Rietmeijer, G. J. Flynn, J.-B. Renard, J.R. Stephens, and E. Zona. "The DUSTER experiment: collection and analysis of aerosol in the high stratosphere". Retrieved 19 February 2009.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ↑ Myhre, Gunnar; Stordal, Frode; Berglen, Tore F.; Sundet, Jostein K.; Isaksen, Ivar S. A. (2004-03-01). "Uncertainties in the Radiative Forcing Due to Sulfate Aerosols". Journal of the Atmospheric Sciences. 61 (5): 485–498. Bibcode:2004JAtS...61..485M. doi:10.1175/1520-0469(2004)061<0485:UITRFD>2.0.CO;2. ISSN 0022-4928. S2CID 55623817.
- ↑ Zhang, Jie; Furtado, Kalli; Turnock, Steven T.; Mulcahy, Jane P.; Wilcox, Laura J.; Booth, Ben B.; Sexton, David; Wu, Tongwen; Zhang, Fang; Liu, Qianxia (22 December 2021). "The role of anthropogenic aerosols in the anomalous cooling from 1960 to 1990 in the CMIP6 Earth system models". Atmospheric Chemistry and Physics. 21 (4): 18609–18627. Bibcode:2021ACP....2118609Z. doi:10.5194/acp-21-18609-2021.
- ↑ "Aerosols and Incoming Sunlight (Direct Effects)". NASA. 2 November 2010.
- ↑ "Stratospheric Injections Could Help Cool Earth, Computer Model Shows". ScienceDaily. 15 September 2006. Retrieved 19 February 2009.
- ↑ Launder B. & J.M.T. Thompson (1996). "Global and Arctic climate engineering: numerical model studies". Phil. Trans. R. Soc. A. 366 (1882): 4039–56. Bibcode:2008RSPTA.366.4039C. doi:10.1098/rsta.2008.0132. PMID 18757275.
- ↑ Crutzen, P. J. (2006). "Albedo Enhancement by Stratospheric Sulfur Injections: A Contribution to Resolve a Policy Dilemma?". Climatic Change. 77 (3–4): 211–220. Bibcode:2006ClCh...77..211C. doi:10.1007/s10584-006-9101-y.
- ↑ Visioni, Daniele; Slessarev, Eric; MacMartin, Douglas G; Mahowald, Natalie M; Goodale, Christine L; Xia, Lili (1 September 2020). "What goes up must come down: impacts of deposition in a sulfate geoengineering scenario". Environmental Research Letters. 15 (9): 094063. Bibcode:2020ERL....15i4063V. doi:10.1088/1748-9326/ab94eb. ISSN 1748-9326.
- ↑ Andrew Charlton-Perez & Eleanor Highwood. "Costs and benefits of geo-engineering in the Stratosphere" (PDF). Archived from the original (PDF) on 14 January 2017. Retrieved 17 February 2009.
- ↑ Trisos, Christopher H.; Geden, Oliver; Seneviratne, Sonia I.; Sugiyama, Masahiro; van Aalst, Maarten; Bala, Govindasamy; Mach, Katharine J.; Ginzburg, Veronika; de Coninck, Heleen; Patt, Anthony (2021). "Cross-Working Group Box SRM: Solar Radiation Modification" (PDF). Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. 2021: 1238. Bibcode:2021AGUFM.U13B..05K. doi:10.1017/9781009157896.007.
- ↑ Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005 (PDF), IUPAC, p. 129, archived (PDF) from the original on 2017-05-18
- ↑ Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005 (PDF), IUPAC, p. 129, archived (PDF) from the original on 2017-05-18

