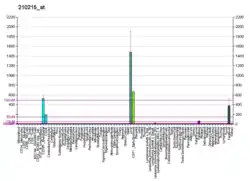
Transferrin receptor 2 (TfR2) is a protein that in humans is encoded by the TFR2 gene.[5][6] This protein is involved in the uptake of transferrin-bound iron into cells by endocytosis, although its role is minor compared to transferrin receptor 1.
Function
This gene is a member of the transferrin receptor-like family and encodes a single-pass type II membrane protein with a protease associated (PA) domain, an M28 peptidase domain and a transferrin receptor-like dimerization domain. This protein mediates cellular uptake of transferrin-bound iron and mutations in this gene have been associated with hereditary hemochromatosis type III. Alternatively spliced variants which encode different protein isoforms have been described; however, not all variants have been fully characterized.[7]
See also
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000106327 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000029716 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ Glockner G, Scherer S, Schattevoy R, Boright A, Weber J, Tsui LC, Rosenthal A (Dec 1998). "Large-Scale Sequencing of Two Regions in Human Chromosome 7q22: Analysis of 650 kb of Genomic Sequence around the EPO and CUTL1 Loci Reveals 17 Genes". Genome Res. 8 (10): 1060–73. doi:10.1101/gr.8.10.1060. PMC 310788. PMID 9799793.
- ↑ Mattman A, Huntsman D, Lockitch G, Langlois S, Buskard N, Ralston D, Butterfield Y, Rodrigues P, Jones S, Porto G, Marra M, De Sousa M, Vatcher G (Jul 2002). "Transferrin receptor 2 (TfR2) and HFE mutational analysis in non-C282Y iron overload: identification of a novel TfR2 mutation". Blood. 100 (3): 1075–7. doi:10.1182/blood-2002-01-0133. hdl:10400.16/826. PMID 12130528.
- ↑ "Entrez Gene: TFR2 transferrin receptor 2".
Further reading
- Subramaniam VN, Summerville L, Wallace DF (2003). "Molecular and cellular characterization of transferrin receptor 2". Cell Biochem. Biophys. 36 (2–3): 235–9. doi:10.1385/CBB:36:2-3:235. PMID 12139409. S2CID 278321.
- Trinder D, Baker E (2003). "Transferrin receptor 2: a new molecule in iron metabolism". Int. J. Biochem. Cell Biol. 35 (3): 292–6. doi:10.1016/S1357-2725(02)00258-3. PMID 12531241.
- Franchini M (2006). "Hereditary iron overload: update on pathophysiology, diagnosis, and treatment". Am. J. Hematol. 81 (3): 202–9. doi:10.1002/ajh.20493. PMID 16493621. S2CID 40950367.
- Deicher R, Hörl WH (2006). "New insights into the regulation of iron homeostasis". Eur. J. Clin. Invest. 36 (5): 301–9. doi:10.1111/j.1365-2362.2006.01633.x. PMID 16634833. S2CID 1726652.
- Feder JN, Penny DM, Irrinki A, et al. (1998). "The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding". Proc. Natl. Acad. Sci. U.S.A. 95 (4): 1472–7. Bibcode:1998PNAS...95.1472F. doi:10.1073/pnas.95.4.1472. PMC 19050. PMID 9465039.
- Kawabata H, Yang R, Hirama T, et al. (1999). "Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family". J. Biol. Chem. 274 (30): 20826–32. doi:10.1074/jbc.274.30.20826. PMID 10409623.
- Bennett MJ, Lebrón JA, Bjorkman PJ (2000). "Crystal structure of the hereditary haemochromatosis protein HFE complexed with transferrin receptor". Nature. 403 (6765): 46–53. doi:10.1038/47417. PMID 10638746. S2CID 4427272.
- Kawabata H, Germain RS, Vuong PT, et al. (2000). "Transferrin receptor 2-alpha supports cell growth both in iron-chelated cultured cells and in vivo". J. Biol. Chem. 275 (22): 16618–25. doi:10.1074/jbc.M908846199. PMID 10748106.
- Camaschella C, Roetto A, Calì A, et al. (2000). "The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22". Nat. Genet. 25 (1): 14–5. doi:10.1038/75534. PMID 10802645. S2CID 9227077.
- West AP, Bennett MJ, Sellers VM, et al. (2001). "Comparison of the interactions of transferrin receptor and transferrin receptor 2 with transferrin and the hereditary hemochromatosis protein HFE" (PDF). J. Biol. Chem. 275 (49): 38135–8. doi:10.1074/jbc.C000664200. PMID 11027676. S2CID 14295069.
- Roetto A, Totaro A, Piperno A, et al. (2001). "New mutations inactivating transferrin receptor 2 in hemochromatosis type 3". Blood. 97 (9): 2555–60. doi:10.1182/blood.V97.9.2555. PMID 11313241. S2CID 36015072.
- Kawabata H, Nakamaki T, Ikonomi P, et al. (2001). "Expression of transferrin receptor 2 in normal and neoplastic hematopoietic cells". Blood. 98 (9): 2714–9. doi:10.1182/blood.V98.9.2714. PMID 11675342.
- Fleming RE, Ahmann JR, Migas MC, et al. (2002). "Targeted mutagenesis of the murine transferrin receptor-2 gene produces hemochromatosis". Proc. Natl. Acad. Sci. U.S.A. 99 (16): 10653–8. doi:10.1073/pnas.162360699. PMC 125003. PMID 12134060.
- Hofmann WK, Tong XJ, Ajioka RS, et al. (2002). "Mutation analysis of transferrin-receptor 2 in patients with atypical hemochromatosis". Blood. 100 (3): 1099–100. doi:10.1182/blood-2002-04-1077. PMID 12150153.
- Deaglio S, Capobianco A, Calì A, et al. (2003). "Structural, functional, and tissue distribution analysis of human transferrin receptor-2 by murine monoclonal antibodies and a polyclonal antiserum". Blood. 100 (10): 3782–9. doi:10.1182/blood-2002-01-0076. PMID 12393650.
- Vogt TM, Blackwell AD, Giannetti AM, et al. (2003). "Heterotypic interactions between transferrin receptor and transferrin receptor 2". Blood. 101 (5): 2008–14. doi:10.1182/blood-2002-09-2742. PMID 12406888.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.