 | |
| Names | |
|---|---|
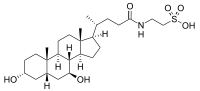
| IUPAC name
2-(3α,7β-Dihydroxy-5β-cholan-24-amido)ethane-1-sulfonic acid | |
| Systematic IUPAC name
2-{(4R)-4-[(1R,3aS,3bR,4S,5aS,7R,9aS,9bS,11aR)-4,7-Dihydroxy-9a,11a-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-1-yl]pentanamido}ethane-1-sulfonic acid | |
| Other names
Tauroursodeoxycholic acid; TUDCA; 3α,7β-Dihydroxy-5β-cholanoyltaurine; UR 906; Ursodeoxycholyltaurine; Taurursodiol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C26H45NO6S | |
| Molar mass | 499.71 g·mol−1 |
| Pharmacology | |
| A05AA05 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Ursodoxicoltaurine is the international nonproprietary name (INN) for the pharmaceutical form of tauroursodeoxycholic acid (TUDCA). It is also known as taurursodiol. Tauroursodeoxycholic acid is a naturally occurring hydrophilic bile acid which is the taurine conjugated form of ursodeoxycholic acid (UDCA). Humans have only trace amounts of tauroursodeoxycholic acid but bears have large amounts of tauroursodeoxycholic acid and ursodeoxycholic acid in their bile.[1]
Synthesis
Bile acids are naturally synthesized from cholesterol in the liver and are conjugated with specific amino-acids, specifically taurine. Bear bile contains several bile acids including taurochenodeoxycholic acid, ursodeoxycholic acid, and chenodeoxycholic acid.[2] UDCA and its taurine conjugates comprise about 47% of the bile in American black bears and up to 76% in Asiatic bears.[1][3] Ursodeoxycholic acid and tauroursodeoxycholic acid were first chemically synthesized in 1954 in Japan.[1] Ursodeoxycholic acid is produced in several countries for the treatment of gallstones and primary biliary cholangitis.[4]
Medical uses
In Canada and the United States, ursodoxicoltaurine, in combination with sodium phenylbutyrate, is indicated for the treatment of amyotrophic lateral sclerosis (ALS).[5][6]
Cellular mechanisms
Apoptosis is largely influenced by the mitochondria. If the mitochondria are distressed, they release cytochrome C (cyC) and calcium which activate caspases to propagate a cascade of cellular mechanisms to cause apoptosis. Tauroursodeoxycholic acid prevents apoptosis with its role in the BAX pathway.[7] Tauroursodeoxycholic acid prevents BAX from being transported to the mitochondria which protects the mitochondria from perturbation and the activation of caspases.[8] Many effects of tauroursodeoxycholic acid appear to be dependent on the activation of the cell membrane receptors TGR5, S1PR2 and α5β1-Integrin.[8]
Tauroursodeoxycholic acid also acts as a chemical chaperone to help maintain the stability and correct folding of proteins.[9]
Research
Ursodoxicoltaurine has been shown to reduce apoptosis and to have protective effects in neurodegenerative diseases and the eye, particularly for retinal degenerative disorders.[9][10]
Studies have shown that tauroursodeoxycholic acid has neuroprotective actions based on its potent ability to inhibit apoptosis, attenuate oxidative stress, and reduce endoplasmic reticulum stress in different experimental models of these illnesses.[8]
Studies have shown protective effects of tauroursodeoxycholic acid in eye diseases.[10]
Photoreceptor cells
A study examined the effects of tauroursodeoxycholic acid on cones, in relation to retinitis pigmentosa (RP), a disease in which retinal rods and cones undergo apoptosis. Mice models were used, a wild-type and a mutant RP model, rd10. Both models were injected with tauroursodeoxycholic acid every 3 days from post-natal day 6 (p6) to p30 and compared to the vehicle. Electroretinography (ERG), photoreceptor cell counts, cone photoreceptor nuclei counts, and TUNEL labeling were all analyzed to show the effects of ursodoxicoltaurine. The dark-adapted and light-adapted ERG responses were greater in the ursodoxicoltaurine treated mouse than the vehicle treated mouse. Ursodoxicoltaurine treated mice also had more photoreceptor counts, yet non-altered retinal morphology or function. Even at P30, a stage where rod and cone function is usually greatly diminished in the rd10 mouse model, the photoreceptor function was protected.[11]
Another study, from the Department of Ophthalmology at Johns Hopkins University, in Baltimore, Maryland, saw similar effects in two components of bile, bilirubin and ursodoxicoltaurine, in relation to RP. Oxidative stress and prolonged light exposure were studied in rd10 mice and albino mice. In rd10 mice, intraperitoneal injections of bilirubin or ursodoxicoltaurine were given every 3 days starting at P6. This caused a considerable preservation in cone cell amount and function at P50, and a modest rod cell amount at P30. In the albino mice models, intraperitoneal injections of bilirubin or ursodoxicoltaurine were given prior to prolonged light exposure. Both treatments had positive effects on the health of the mouse retina, including a reduced accumulation of superoxide radicals, rod cell death, and disruption of cone inner and outer segments. The findings of the study are elucidating optimized conditions for RP treatment.[12]
Choroidal neovascularization
A study done at the Department of Ophthalmology at Seoul National University College of Medicine examined the effects of ursodoxicoltaurine and UDCA on laser-treated choroids of rat models. Argon lasers were used to induce choroidal neovascularization (CNV) in rat models. Ursodoxicoltaurine and UDCA were injected intraperitoneally 24 hours before and daily after the laser treatment. Fourteen days after laser-treatment, the eyes were examined for effects. Fluorescein angiography showed lower leakage from the CNV in UDCA and ursodoxicoltaurine treated groups than the control group. Additionally, vascular endothelial growth factor (VEGF) levels in the retina were examined and showed lower levels in the ursodoxicoltaurine treated group compared to the control group, whereas no effect in the UDCA treated group. ursodoxicoltaurine and UDCA may suppress CNV formation, which may be associated with its anti-inflammatory effects.[13]
Synaptic connectivity
A study from the Department of Physiology in University of Alicante, in Alicante, Spain, shows the effects of ursodoxicoltaurine in the P23H transgenic rat, a model of autosomal dominant retinitis pigmentosa. The transgenic rats were injected with ursodoxicoltaurine once a week starting from P21 until P120, along with vehicle-administered controls. At P120, the functionality of the retina was examined via ERG and immunoflourescent microscopy. The amplitude of the a- and b- waves were considerably higher in ursodoxicoltaurine treated rats, compared to the control group. Photoreceptor density in the center of the retina was three-fold greater in ursodoxicoltaurine treated rats. Also, TUNEL results showed smaller amounts of TUNEL-positive cells. The synaptic contacts amongst photoreceptor cells, bipolar cells, and horizontal cells were preserved in the ursodoxicoltaurine treated P23H rats. Additionally, the synaptic terminals in the outer plexiform layer were of greater density that in control rats. The neuroprotective effects of ursodoxicoltaurine are not only preserving retinal morphology and function, but also its synaptic contacts, a potentially useful aspect in delaying RP.[14]
Medical uses
Tauroursodeoxycholic acid has been suggested to have potential application in heart disease, Huntington's disease, Parkinson's disease, amyotrophic lateral sclerosis and stroke in view of its ability to reduce apoptotic effects.[9][8][15][7]
References
- 1 2 3 Hagey LR, Crombie DL, Espinosa E, Carey MC, Igimi H, Hofmann AF (November 1993). "Ursodeoxycholic acid in the Ursidae: biliary bile acids of bears, pandas, and related carnivores". Journal of Lipid Research. 34 (11): 1911–7. doi:10.1016/S0022-2275(20)35109-9. PMID 8263415.
- ↑ Luo Q, Chen Q, Wu Y, Jiang M, Chen Z, Zhang X, Chen H (2010). "[Chemical constituents of bear bile]". Zhongguo Zhong Yao Za Zhi. 35 (18): 2416–2419. PMID 21141490.
- ↑ Boatright JH, Nickerson JM, Moring AG, Pardue MT (September 2009). "Bile acids in treatment of ocular disease". Journal of Ocular Biology, Diseases, and Informatics. 2 (3): 149–159. doi:10.1007/s12177-009-9030-x. PMC 2798994. PMID 20046852.
- ↑ Carey EJ, Ali AH, Lindor KD (October 2015). "Primary biliary cirrhosis". Lancet. 386 (10003): 1565–75. doi:10.1016/S0140-6736(15)00154-3. PMID 26364546. S2CID 44811681.
- ↑ "Albrioza monograph" (PDF). 1 June 2022. Archived (PDF) from the original on 14 June 2022. Retrieved 14 June 2022.
- ↑ "Relyvrio- sodium phenylbutyrate/taurursodiol powder, for suspension". DailyMed. 30 September 2022. Retrieved 3 January 2023.
- 1 2 Rivard AL, Steer CJ, Kren BT, Rodrigues CM, Castro RE, Bianco RW, Low WC (2007). "Administration of tauroursodeoxycholic acid (TUDCA) reduces apoptosis following myocardial infarction in rat". The American Journal of Chinese Medicine. 35 (2): 279–95. doi:10.1142/S0192415X07004813. PMID 17436368.
- 1 2 3 4 Zangerolamo L, Vettorazzi JF, Rosa LR, Carneiro EM, Barbosa HC (May 2021). "The bile acid TUDCA and neurodegenerative disorders: An overview". Life Sciences. 272: 119252. doi:10.1016/j.lfs.2021.119252. PMID 33636170. S2CID 232066323.
- 1 2 3 Khalaf K, Tornese P, Cocco A, Albanese A (June 2022). "Tauroursodeoxycholic acid: a potential therapeutic tool in neurodegenerative diseases". Translational Neurodegeneration. 11 (1): 33. doi:10.1186/s40035-022-00307-z. PMC 9166453. PMID 35659112.
- 1 2 Daruich A, Picard E, Boatright JH, Behar-Cohen F (2019). "Review: The bile acids urso- and tauroursodeoxycholic acid as neuroprotective therapies in retinal disease". Molecular Vision. 25: 610–624. PMC 6817734. PMID 31700226.
- ↑ Phillips Joe M; Walker Tiffany A; Choi Hee-Young; Faulkner Amanda E; Kim Moon K; Sidney Sheree S; Boyd Amber P; Nickerson John M; Boatright Jeffrey H; Pardue Machelle T (2008). "Tauroursodeoxycholic acid preservation of photoreceptor structure and function in the rd10 mouse through postnatal day 30". Invest Ophthalmol Vis Sci. 49 (5): 2148–2155. doi:10.1167/iovs.07-1012. PMC 2626193. PMID 18436848.
- ↑ Oveson BC, Iwase T, Hackett SF, Lee SY, Usui S, Sedlak TW, Snyder SH, Campochiaro PA, Sung JU (January 2011). "Constituents of bile, bilirubin and TUDCA, protect against oxidative stress-induced retinal degeneration". Journal of Neurochemistry. 116 (1): 144–53. doi:10.1111/j.1471-4159.2010.07092.x. PMC 4083853. PMID 21054389.
- ↑ Woo SJ, Kim JH, Yu HG (2010). "Ursodeoxycholic acid and tauroursodeoxycholic acid suppress choroidal neovascularization in a laser-treated rat model". J Ocul Pharmacol Ther. 26 (3): 223–229. doi:10.1089/jop.2010.0012. PMID 20565307.
- ↑ Fernández-Sánchez L, Lax P, Pinilla I, Martín-Nieto J, Cuenca N (2011). "Tauroursodeoxycholic Acid (TUDCA) Prevents Retinal Degeneration in Transgenic P23H Rats". Invest Ophthalmol Vis Sci. 52 (8): 4998–5008. doi:10.1167/iovs.11-7496. PMID 21508111.
- ↑ Vang S, Longley K, Steer CJ, Low WC (May 2014). "The Unexpected Uses of Urso- and Tauroursodeoxycholic Acid in the Treatment of Non-liver Diseases". Global Advances in Health and Medicine. 3 (3): 58–69. doi:10.7453/gahmj.2014.017. PMC 4030606. PMID 24891994.