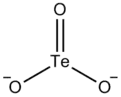
 | |
| Names | |
|---|---|
| Systematic IUPAC name | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| 100741 | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| O3Te2− | |
| Molar mass | 175.6 g mol−1 |
| Conjugate acid | Tellurous acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The tellurite ion is TeO2−
3. A tellurite (compound), for example sodium tellurite, is a compound that contains this ion. They are typically colorless or white salts, which in some ways are comparable to sulfite.[3] A mineral with the formula TeO2 is called tellurite.
Structure and reactions
Tellurite dianion is pyramidal, like selenite and sulfite. The anion has C3v symmetry.
Tellurites can be reduced to elemental tellurium by electrolysis or a strong reducing agent. When fused with nitrate salts, tellurite salts oxidize to tellurates (TeO2−
4).
Upon acidification of aqueous solutions of tellurite salts, solid hydrated tellurium dioxide (TeO2) precipitates. This reaction allows the separation of tellurium from selenium since selenous acid remains soluble at low pH. The intermediate in the protonation occurs at oxygen to give [TeO2(OH)]−.
Uses
Potassium tellurite (K2TeO3) is used together with agar as part of a selective medium for growth of some bacteria (Clauberg medium). Corynebacteria and some other species reduce TeO2−
3 to elemental Te, which stains the bacteria black.
See also
- List of tellurites
References
- ↑ "Tellurous Acid - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- ↑ "Tellurite (CHEBI:30477)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
Further reading
- M. R. Masson, H. D. Lutz and B. Engelen (eds.) "Sulfites, Selenites and Tellurites", Pergamon Press, Oxford, 1986.