 | |
| Names | |
|---|---|
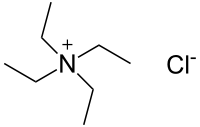
| Preferred IUPAC name
N,N,N-Triethylethanaminium chloride | |
| Other names
Tetraethylammonium chloride N,N,N,N-Tetraethylammonium chloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.243 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| [N(CH2CH3)4]Cl | |
| Molar mass | 165.71 g·mol−1 |
| Appearance | Colorless deliquescent crystalline solid |
| Density | 1.08 g/cm3[1] |
| Melting point | 360 °C (680 °F; 633 K) tetrahydrate[1] |
| highly soluble | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
65 mg/kg (mouse, i.p.) 900 mg/kg (mouse, p.o.) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Tetraethylammonium chloride (TEAC) is a quaternary ammonium compound with the chemical formula [N(CH2CH3)4]+Cl−, sometimes written as [NEt4]Cl. In appearance, it is a hygroscopic, colorless, crystalline solid. It has been used as the source of tetraethylammonium ions in pharmacological and physiological studies, but is also used in organic chemical synthesis.
Preparation and structure
TEAC is produced by alkylation of triethylamine with ethyl chloride.[2]
TEAC exists as either of two stable hydrates, the monohydrate and tetrahydrate.[3] The crystal structure of TEAC·H2O has been determined,[4] as has that of the tetrahydrate, TEAC·4H2O.[5]
Details for the preparation of large, prismatic crystals of TEAC·H2O are given by Harmon and Gabriele, who carried out IR-spectroscopic studies on this and related compounds.[6] These researchers have also pointed out that, although freshly-purified TEAC·H2O is free of triethylamine hydrochloride, small quantities of this compound form on heating of TEAC as the result of a Hofmann elimination:
- Cl− + [N(CH2CH3)4]+ → HCl + H2C=CH2 + N(CH2CH3)3
Synthetic Applications
To a large extent, the synthetic applications of TEAC resemble those of tetraethylammonium bromide (TEAB) and tetraethylammonium iodide (TEAI), although one of the salts may be more efficacious than another in a particular reaction. For example, TEAC produces better yields than TEAB or TEAI as a co-catalyst in a reaction to prepare diarylureas from arylamines, nitroaromatics and carbon monoxide.[7]
In other examples, such as the following, TEAC is not as effective as TEAB or TEAI:
- 2-Hydroxyethylation (attachment of −CH2−CH2−OH) by ethylene carbonate of carboxylic acids and certain heterocycles bearing an acidic N-H.[8]
- Phase-transfer catalyst in geminal di-alkylation of fluorene, N,N-dialkylation of aniline and N-alkylation of carbazole using aqueous sodium hydroxide and alkyl halides.[9]
Biology
In common with tetraethylammonium bromide and tetraethylammonium iodide, TEAC has been used as a source of tetraethylammonium ions for numerous clinical and pharmacological studies, which are covered in more detail under the entry for Tetraethylammonium. Briefly, TEAC has been explored clinically for its ganglionic blocking properties,[10] although it is now essentially obsolete as a drug, and it is still used in physiological research for its ability to block K+ channels in various tissues.[11][12]
Toxicity
The toxicity of TEAC is primarily due to the tetraethylammonium ion, which has been studied extensively. The acute toxicity of TEAC is comparable to that of tetraethylammonium bromide and tetraethylammonium iodide. These data[13] are provided for comparative purposes; additional details may be found in the entry for Tetraethylammonium.
See also
References
- 1 2 The Merck Index, 10th Ed., p.1316, Rahway: Merck & Co.
- ↑ Roose, Peter; Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (2015). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001.pub2. ISBN 978-3527306732.
- ↑ K. M. Harmon, J. M. Gabriele and J. Harmon (1990). "Hydrogen bonding Part 30. New IR spectra-structure correlations for tetraethylammonium, tetramethylammonium, and N,N-dimethyl-pyrrolidinium fluoride monohydrates, tetraethylammonium chloride monohydrate, and tetramethylammonium hydroxide dihydrate; evidence for a planar (H2O.F−)2 cluster". J. Mol. Struct. 216 53-62.
- ↑ J. H. Loehlin and A. Kvick (1978). "Tetraethylammonium chloride monohydrate". Acta Crystallographica Section B 34 3488–3490.
- ↑ Y.-S. Lam and T. C. W. Mak (1978). "Crystal data for some tetraethylammonium salt hydrates". 11 193.
- ↑ Harmon, Kenneth M.; Gabriele, Julie M. (1981). "Hydrogen bonding. 11. Infrared study of the water-chloride ion cluster in tetraethylammonium chloride hydrate". Inorganic Chemistry. 20 (11): 4013–4015. doi:10.1021/ic50225a087.
- ↑ H. A. Dieck, R. M. Laine and R. F. Heck (1975). "Low-pressure, palladium-catalyzed N,N'-diarylurea synthesis from nitro compounds, amines, and carbon monoxide". J. Org. Chem. 40 2819–2822.
- ↑ T.Yoshino et al. (1977). "Synthetic studies with carbonates. Part 6. Syntheses of 2-hydroxyethyl derivatives by reactions of ethylene carbonate with carboxylic acids or heterocycles in the presence of tetraethylammonium halides or under autocatalytic conditions". J. Chem. Soc., Perkin 1 1266–1272.
- ↑ G. Saikia and P. K. Iyer (2010). "Facile C-H alkylation in water: enabling defect-free materials for optoelectronic devices". J. Org. Chem. 75 2714–2717.
- ↑ G. K. Moe and W. A. Freyburger (1950). "Ganglionic blocking agents". Pharmacol. Rev. 2 61–95.
- ↑ B. Hille (1967). "The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ions". J. Gen. Physiol. 50 1287–1302.
- ↑ C. M. Armstrong and B. Hille (1972). "The inner quaternary ammonium receptor in potassium channels of the node of Ranvier". J. Gen. Physiol. 59 388–400.
- ↑ O. M. Gruhzit, R. A. Fisken and B. J. Cooper (1948). "Tetraethylammonium chloride [(C2H5)4NCl]. Acute and chronic toxicity in experimental animals". J. Pharmacol. Exp. Ther. 92 103–107.