 | |
| Names | |
|---|---|
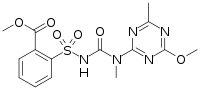
| IUPAC name
Methyl 2-[[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-methylcarbamoyl]sulfamoyl]benzoate | |
| Other names
DPX L5300 | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.100.313 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties[1] | |
| C15H17N5O6S | |
| Molar mass | 395.39 g·mol−1 |
| Appearance | White to off-white solid |
| Density | 1.46 g/cm3 |
| Melting point | 142 |
| 2483 mg/L (20 °C) | |
| log P | 0.38 |
| Acidity (pKa) | 4.65 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Tribenuron in the form of tribenuron-methyl is a sulfonylurea herbicide.[2] Its mode of action is the inhibition of acetolactate synthase, group 2 of the Herbicide Resistance Action Committee's classification scheme.[3][4]
Chemistry
In the 1970s, chemists at DuPont worked extensively on sulfonylurea herbicides, following the invention of this class of herbicides by George Levitt which had led to the commercialisation of chlorsulfuron.[5] Tribenuron (the carboxylic acid) and its methyl ester[6] were first disclosed in general terms in one of Levitt's patents[7] and subsequently the ester was subject to further patenting[8] and selected for development under the code name DPX L5300.[1] In the final step of its synthesis, 2-methoxycarbonylbenzenesulfonyl isocyanate was condensed with 2-methylamino-4-methoxy-6-methyl-1,3,5-triazine to form the sulfonylurea product.[8]
Mode of action
Tribenuron is an herbicide that acts as an acetolactate synthase inhibitor.[2][3] For the purposes of herbicide resistance management, the Herbicide Resistance Action Committee has placed it in group 2 (legacy HRAC Group B).[4]
Applications
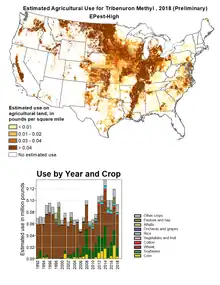
Tribenuron has a broad spectrum of activity on commercially important broadleaf weeds and grasses but at the recommended use rate it is safe to important crops such as wheat. When introduced by DuPont, its recommended application rate was 0.015–0.03 pounds per acre (17–34 g/ha).[9] The estimated use in US agriculture is mapped by the US Geological Service and shows that from 1992 to 2018, up to 120,000 pounds (54,000 kg) were applied each year. The compound is used mainly in wheat but also in pasture.[10]
Physicochemistry
In a clay-water suspension, tribenuron has increased sorption with decreasing pH and even more so with suspended load.[11]
Resistant crops
A tribenuron-resistance transformation has been achieved in watermelon and validated by survival of the als mutants but not the controls, under tribenuron treatment.[12]
Two oilseed type sunflower cultivars have been produced by USDA-ARS by conventional breeding.[13]
References
- 1 2 Pesticide Properties Database. "Tribenuron-methyl". University of Hertfordshire.
- 1 2 Herbicide Resistance Action Committee (HRAC) (2 June 2020). "Global HRAC MOA Classification Working Group Report" (PDF).
- 1 2 Scott Senseman (3 December 2020). "Weed Science Society of America - Herbicide Site of Action (SOA) Classification List".
- 1 2 "Herbicide Classification Master: March 11, 2020". Herbicide Resistance Action Committee (HRAC). 11 March 2020.
- ↑ Bhardwaj, Gaurab (2007). "From Pioneering Invention to Sustained Innovation: The Story of Sulfonylurea Herbicides" (PDF). Chemical Heritage NewsMagazine. 25 (1). Archived from the original (PDF) on 2006-11-18.
- ↑ Weed Science Society of America (WSSA) (2014). "Common and Chemical Names of Herbicides Approved by the Weed Science Society of America". Weed Science. Cambridge University Press (CUP). 62 (4): 679–687. doi:10.1614/0043-1745-62.4.679. ISSN 0043-1745. S2CID 198130304.
- ↑ US patent 4383113, George Levitt, "Agricultural sulfonamides", issued 1983-05-10, assigned to E. I. du Pont de Nemours and Company
- 1 2 US patent 4740234, Gerald E. Lepone, "Herbicidal ortho-carbomethoxysulfonylureas", issued 1988-04-26, assigned to E. I. du Pont de Nemours and Company
- ↑ DuPont (2004). "DuPont Tribenuron Methyl 50SG" (PDF).
- ↑ US Geological Survey. "Estimated Agricultural Use for tribenuron methyl, 2018". Retrieved 2023-01-13.
- ↑ Salbu, Brit [in Norwegian]; Steinnes, Eiliv [in Norwegian] (1992). "Applications of nuclear analytical techniques in environmental research. Plenary lecture". The Analyst. Royal Society of Chemistry (RSC). 117 (3): 243–9. Bibcode:1992Ana...117..243S. doi:10.1039/an9921700243. ISSN 0003-2654. PMID 1580364. p. 248.
- ↑ Wang, Tian; Zhang, Hongyan; Zhu, Hongliang (2019-06-15). "CRISPR technology is revolutionizing the improvement of tomato and other fruit crops". Horticulture Research. Nature Research. 6 (1): 77. doi:10.1038/s41438-019-0159-x. ISSN 2052-7276. PMC 6570646. PMID 31240102.
- ↑ Miller, J.F.; Al‐Khatib, Kassim (2004). "Registration of Two Oilseed Sunflower Genetic Stocks, SURES‐1 and SURES‐2 Resistant to Tribenuron Herbicide". Crop Science. Crop Science Society of America (Wiley). 44 (3): 1037–1038. doi:10.2135/cropsci2004.1037. ISSN 0011-183X. S2CID 85001003.
Further reading
- Kreiner, Julia M.; Stinchcombe, John R.; Wright, Stephen I. (2018-04-29). "Population Genomics of Herbicide Resistance: Adaptation via Evolutionary Rescue". Annual Review of Plant Biology. Annual Reviews. 69 (1): 611–635. doi:10.1146/annurev-arplant-042817-040038. ISSN 1543-5008. PMID 29140727. S2CID 25489201. Supplemental.
