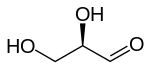
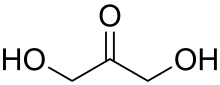
Dihydroxyacetone is a ketotriose because the carbonyl group is the center of the chain.
A triose is a monosaccharide, or simple sugar, containing three carbon atoms. There are only three possible trioses: the two enantiomers of glyceraldehyde, which are aldoses; and dihydroxyacetone, a ketose which is symmetrical and therefore has no enantiomers.[1]
Trioses are important in cellular respiration. During glycolysis, fructose-1,6-bisphosphate is broken down into glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. Lactic acid and pyruvic acid are later derived from these molecules.[2]
References
- ↑ "Trioses - Three Carbon Sugars". Oxford University Press. Retrieved 2011-07-10.
- ↑ "Glycolysis in Detail". Ohio State University at Mansfield. Retrieved 2011-07-10.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.