 | |
| Names | |
|---|---|
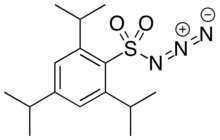
| IUPAC name
N-diazo-2,4,6-tri(propan-2-yl)benzenesulfonamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.124.728 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H23N3O2S | |
| Molar mass | 309.43 g·mol−1 |
| Melting point | 39–44 °C (102–111 °F; 312–317 K) |
| Hazards | |
| GHS labelling:[1] | |
  | |
| Danger | |
| H241, H301 | |
| P210, P234, P240, P261, P264, P264+P265, P270, P271, P280, P301+P316, P302+P352, P304+P340, P305+P351+P338, P319, P321, P330, P332+P317, P337+P317, P362+P364, P370+P380+P375+P378, P403, P403+P233, P405, P410, P411, P420, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
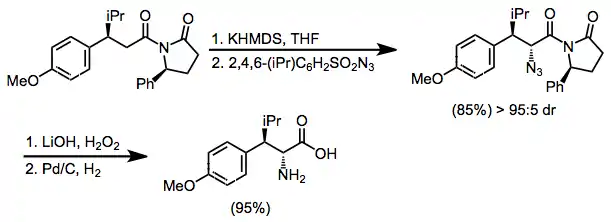
2,4,6-Triisopropylbenzenesulfonyl azide (trisyl azide) is an organic chemical used as a reagent to supply azide for electrophilic amination reactions, such as for the asymmetric synthesis of unnatural amino acids.[2] Introduction of an azide on the α carbon of carboxylic acid derivative using trisyl azide is an efficient alternative to electrophilic halogenation followed by nucleophilic substitution using anionic azide. Using an oxazolidinone as chiral auxiliary typically gives good induction of the stereochemistry at the α position. Subsequent reduction converts the α-azide to an α-amine.
References
- ↑ "2,4,6-Triisopropylbenzenesulfonyl azide". pubchem.ncbi.nlm.nih.gov. Retrieved 1 May 2022.
- ↑ Evans, David A.; Britton, Thomas C. (1987). "Electrophilic azide transfer to chiral enolates. A general approach to the asymmetric synthesis of α-amino acids". J. Am. Chem. Soc. 109 (22): 6881–3. doi:10.1021/ja00256a069.
Further reading
- Calmes, M.; Daunis, J. (1999). "How to build optically active α-amino acids". Amino Acids. 16 (3–4): 215–250. doi:10.1007/BF01388170. PMID 10399014. S2CID 36591079.
- Han, Y.; Lin, J.; Liao, S.; Qiu, W.; Cai, C.; Hruby, V.J. (2002). "Stereoselective synthesis of highly topographically constrained β-isopropyl substituted aromatic amino acids". In Tam, J.P.; Kaumaya, P.T.P. (eds.). Peptides Frontiers of Peptide Science. American Peptide Symposia. Vol. 5. Springer. pp. 241–243. doi:10.1007/0-306-46862-X_98. ISBN 0-7923-5160-6.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.