The XMAP215/Dis1 family is a highly conserved group of microtubule-associated proteins (MAPs) in eukaryotic organisms.[1] These proteins are unique MAPs because they primarily interact with the growing-end (plus-end) of microtubules. This special property classifies this protein family as plus-end tracking proteins (+TIPs).[2]
Structure
The basic structure of the protein family consists of TOG (Tumor Overexpressed Gene) domains, ranging from 2-5 units. The family is categorized into three groups based on the number of TOG domains that specific protein contains. Higher eukaryotic organisms, categorized in the first group, contain five, N-terminus TOG domains and a variable region that connects to a C-terminal domain.[3] These domains are highly conserved monomeric sequences. The second group consists of only the Caenorhabditis elegans protein zyg-9, which has three TOG domains.[3] It is similar, though, to higher eukaryotes because of its variable region and C-terminal domain. The third group consists of lower eukaryotic organisms, mainly yeast, that contain only two TOG domains and a coiled-coil domain.[3]
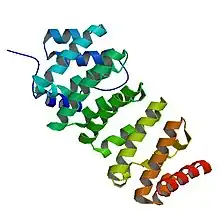
Thorough analysis of the TOG3 domain in zyg-9 provides a basic understanding of this domain that is conserved throughout all members of the XMAP215/Dis1 family.[3] Each domain consists of six HEAT (Huntingtin, Elongation factor 3, the PR65/A subunit of protein phosphatase 2A and the lipid kinase Tor) repeat units that are adjacently aligned. Each HEAT molecule consists of two α helices that are connected by a single loop.[3] These α helices form the wide, flat surface of the domain. The loops between HEAT repeats and between individual α helices run along the short side of the domain. This short region is necessary for binding to tubulin. An additional HEAT repeat, localized between the first and second HEAT repeat, is exclusive to the TOG3 domain in zyg-9 and the TOG5 domains in the first group family proteins.[3]
The C-terminal end of the protein has group-specific characteristics. In the third protein group, the coiled-coil domain is essential for dimerization in simple eukaryotes.[3] This is because simple eukaryotes such as yeast produce proteins in dimers. In first and second groups, the C-terminal domain is known to interact with transforming acidic coiled-coil protein 3 (TACC3), which transports the protein to the centrosomes during mitosis.[4]
Function
Mechanism model
XMAP215/Dis1 proteins can add or remove tubulin dimers in a two-step process. XMAP215 has been shown to bind to tubulin in a 1:1 complex, meaning that XMAP215 might not bind multiple tubulin dimers at once.[5] The αβ-tubulin dimer is known to interact with at least TOG domain, TOG1, which tightly binds inside the bend of the tubulin dimer and is also found beyond the direct plus-end of the microtubule.[6] The tubulin then “straightens,” which forms a weak interaction with TOG1. TOG2, however, can form a tight bind to straight tubulin. Much like a hand-off, TOG1 releases the dimer, which then binds to TOG2. TOG2 then integrates the tubulin dimer into the lattice, extending the microtubule.[6]
Microtubule function
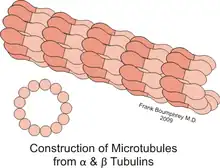
XMAP215/Dis1 family proteins promote both growth and reduction of microtubule length, depending on the concentration of free tubulin; this is known as dynamic instability.[1] Protein behavior is also cell-cycle dependent. Reducing ch-TOG expression leads to improper alignment of the chromosomes during metaphase.[7] One study suggests that in Schizosaccharomyces pombe, the protein Cdc2 regulates Dis1 through phosphorylation and dephosphorylation during metaphase and anaphase. Phosphorylating Dis1 leads to localization at the kinetochores during metaphase, whereas dephosphorylation during anaphase leads to an accumulation of Dis1 on microtubule spindles.[8] In Drosophila, the family member Mini spindles (Msps) is essential for maintaining the integrity of mitotic spindles, which are important for separating chromosomes during mitosis. Reducing Msps activity creates short microtubules, which describes the name of the gene.[9] Msps is also important during oogenesis. When oocytes are depleted of Msps expression, bicoid mRNA localization is less efficient during early stages of oogenesis, but then completely dispersed later in development.[10] Msps is not only responsible for transporting bicoid mRNA throughout the cell, but it also localizes mRNA to the anterior (head) end of the oocyte[10] Additionally, this gene is critical for the organization of tubular endoplasmic reticulum and in Exuperantia protein localization. Exuperantia is necessary for accumulating bicoid mRNA in the head region of the oocyte.[11] Another key function of XMAP215 in microtubule dynamics is in the regulation of axon guidance.[12] This is when microtubules extend into or retract from the axonal growth cone, which guides movement by receiving concentrated signaling cues.[13] In Drosophila, Msps promotes microtubule dynamics in axonal guidance at the embryonic ventral nerve cord midline.[14]
Interactions with plus-end tracking proteins (+TIPs)
Plus-end tracking proteins are enzymes that localize and interact at the plus-end of microtubules. When tagged with green fluorescent protein (GFP), +TIPs can be visualized and tracked in the direction of microtubule growth. As a +TIP, XMAP215/Dis1 family proteins interact with other +TIPs.[2]
EB1
In Xenopus, XMAP215 and EB1 have been reported to interact with each other. While XMAP215 functions to both grow and shrink the microtubule, EB1 is only present during growth.[15] Alone, these proteins have mild effects on microtubule growth. Together, these proteins act in synergy and lengthen microtubules at a much greater rate. Without XMAP215, EB1 does not have a tubulin polymerase that can efficiently construct the microtubule plus-end with free tubulin. Without EB1, XMAP215 continues to add tubulin to the plus-end, but the integrity of the microtubule lattice becomes compromised. This is because EB1 binds to the microtubule lattice as a stabilizer to keep the tubulin straight.[15]
Members
Group 1 (5 TOG domains)
XMAP215: Xenopus Microtubule-associated protein, found in Xenopus species. The number 215 refers to the size of the protein, which is 215 kDa. This protein was discovered in 1987 through the investigation of microtubule regulation in Xenopus oocytes.[16] In 2008, the protein was identified as a plus-end microtubule polymerase.[5]
ch-Tog: colonic and hepatic Tumour Overexpressing Gene, found in Homo sapiens. It was first identified in humans in 1996 as an overexpressed gene in tumors, but was recognized for its plus-end microtubule regulation in 1998.[17]
Msps: Mini spindles. This protein is found in Drosophila species. This protein was discovered in 1999.[9]
DdCP224: Dictyostelium discoideum Centrosomal Protein. This protein's size is approximately 224 kDa. It was detected in 2000 through immunoscreening of DNA libraries for centrosomal proteins.[18]
Mor1: microtubule organisation gene 1. Found in Arabidopsis thaliana. This protein was discovered in 2001 as an organizer of cortical microtubules[19]
Group 2 (3 TOG domains)
zyg-9: zygotic defective mutant, found in C. elegans. In 1976, this gene was identified when zygotes, with such a mutation, failed to hatch. Zyg-9 was identified as a microtubule regulator in 1980.[20]
Group 3 (2 TOG domains)
alp14/Dis1: altered polarity/Defect in sister chromatid disjoining. These proteins are found in S. pombe. Dis1 is the preferred homologue in colder temperatures, while alp14 is preferred in higher temperatures. Dis1 was recognized in 1988, whereas its homologue alp14 was identified in 2001[20]
Stu2p: suppressors of a tubulin mutation. This protein is found in Saccharomyces cerevisiae. It was discovered in 1997 through a screen and was found to influence microtubule regulation.[21] AlpA: alkaline phosphotase, found in Aspergillus nidulans. In 2007, this protein was identified to interact with microtubule plus ends and also localize at spindle bodies, which is characteristic of XMAP215/Dis1 family proteins.[22]
References
- 1 2 Kinoshita, Kazuhisa; Bianca Habermann and Anthony Hyman (June 2002). "XMAP215: a key component of the dynamic microtubule cytoskeleton". Trends in Cell Biology. 12 (6): 267–273. doi:10.1016/S0962-8924(02)02295-X. PMID 12074886.
- 1 2 Galjart, Niels (June 2010). "Plus-End-Tracking Proteins and Their Interactions at Microtubule Ends". Current Biology. 20 (12): R528–37. doi:10.1016/j.cub.2010.05.022. PMID 20620909. S2CID 17558620.
- 1 2 3 4 5 6 7 Al-Bassam, Jawdat; Nicholas Larsen; Anthony Hyman; Stephen Harrison (March 2007). "Crystal Structure of a TOG Domain: Conserved Features of XMAP215/Dis1-Family TOG Domains and Implications for Tubulin Binding". Structure. 15 (3): 355–362. doi:10.1016/j.str.2007.01.012. PMID 17355870.
- ↑ Kinoshita, Kazuhisa; Tim L. Noetzel; Laurence Pelletier; Karl Mechtler; David N. Drechsel; Anne Schwager; Mike Lee; Jordan W. Raff; Anthony A. Hyman (September 19, 2005). "Aurora A phosphorylation of TACC3/maskin is required for centrosome-dependent microtubule assembly in mitosis". Journal of Cell Biology. 170 (7): 1047–1055. doi:10.1083/jcb.200503023. PMC 2171544. PMID 16172205.
- 1 2 Brouhard, Gary; Jeffrey Stear; Tim Noetzel; Jawdat Al-Bassam; Kazuhisa Kinoshita; Stephen Harrison; Jonathon Howard; Anthony Hyman (January 11, 2008). "XMAP215 is a processive microtubule polymerase". Cell. 132 (1): 79–88. doi:10.1016/j.cell.2007.11.043. PMC 2311386. PMID 18191222.
- 1 2 Ayaz, Pelin; Xuecheng Ye; Patrick Huddleston; Chad Brautigam; Luke Rice (August 2012). "A TOG:ab-tubulin Complex Structure Reveals Conformation-Based Mechanisms for a Microtubule Polymerase". Science. 337 (6096): 857–60. Bibcode:2012Sci...337..857A. doi:10.1126/science.1221698. PMC 3734851. PMID 22904013.
- ↑ Gergely, Fanni; Viji Daviam; Jordan Raff (2003). "The ch-TOG/XMAP215 protein is essential for spindle pole organization in human somatic cells". Genes & Development. 17 (3): 336–41. doi:10.1101/gad.245603. PMC 195983. PMID 12569123.
- ↑ Keita, Aoki; Yukinobu Nakaseko; Kazuhisa Kinoshita; Gohta Goshima; Mitsuhiro Yanagida (August 2006). "Cdc2 Phosphorylation of the Fission Yeast Dis1 Ensures Accurate Chromosome Segregation". Current Biology. 16 (16): 1627–1635. doi:10.1016/j.cub.2006.06.065. PMID 16920624.
- 1 2 Cullen, C. Fiona; Peter Deák; David Glover; Hiroyuki Ohkura (September 1999). "A Gene Encoding a Conserved Microtubule-Associated Protein Required for the Integrity of the Mitotic Spindle in Drosophila" (PDF). Journal of Cell Biology. 146 (5): 1005–1018. doi:10.1083/jcb.146.5.1005. PMC 2169485. PMID 10477755.
- 1 2 Moon, Woongjoon; Tulle Hazelrigg (November 2004). "The Drosophila Microtubule-Associated Protein Mini Spindles Is Required for Cytoplasmic Microtubules in Oogenesis". Current Biology. 14 (21): 1957–1961. doi:10.1016/j.cub.2004.10.023. PMID 15530399.
- ↑ Pokrywka, Nancy; Anna Payne-Tobin; Kathleen Raley-Susman; Sasha Swartzman (May 2009). "Microtubules, the ER and Exu: New associations revealed by analysis of mini spindles mutations". Mechanisms of Development. 126 (5–6): 289–300. doi:10.1016/j.mod.2009.03.002. PMC 2731561. PMID 19303437.
- ↑ Lowery, Laura Anne; Alina Stout; Anna E Faris; Liya Ding; Michelle A Baird; Michael Davidson; Gaudenz Danuse; David Van Vector (December 2013). "Growth cone-specific functions of XMAP215 in restricting microtubule dynamics and promoting axonal outgrowth" (PDF). Neural Development. 8: 22. doi:10.1186/1749-8104-8-22. PMC 3907036. PMID 24289819.
- ↑ Lowery, Laura Anne; David Van Vector (May 2009). "The trip of the tip: understanding the growth cone machinery". Nature Reviews Molecular Cell Biology. 10 (5): 332–43. doi:10.1038/nrm2679. PMC 2714171. PMID 19373241.
- ↑ Lowery, L. A.; Lee, H.; Lu, C.; Murphy, R.; Obar, R. A.; Zhai, B.; Schedl, M.; Van Vactor, D.; Zhan, Y. (2010). "Parallel Genetic and Proteomic Screens Identify Msps as a CLASP-Abl Pathway Interactor in Drosophila". Genetics. 185 (4): 1311–1325. doi:10.1534/genetics.110.115626. ISSN 0016-6731. PMC 2927758. PMID 20498300.
- 1 2 Zanic, Marija; Per Widlund; Anthony Hyman; Jonathon Howard (June 2013). "Synergy between XMAP215 and EB1 increases microtubule growth rates to physiological levels". Nature Cell Biology. 15 (6): 688–93. doi:10.1038/ncb2744. PMID 23666085. S2CID 3025200.
- ↑ Gard, David; Marc Kirschner (November 1987). "A Microtubule-associated Protein from Xenopus Eggs That Specifically Promotes Assembly at the Plus-End". The Journal of Cell Biology. 105 (5): 2203–2215. doi:10.1083/jcb.105.5.2203. PMC 2114854. PMID 2890645.
- ↑ Charrasse, Sophie; Marianne Schroeder; Cécile Gauthier-Rouviere; Fabrice Ango; Lynne Cassimeris; David. L. Gard; Christian Larroque (April 1998). "The TOGp protein is a new human microtubule-associated protein homologous to the Xenopus XMAP215". Journal of Cell Science. 111 ( Pt 10) (10): 1371–83. doi:10.1242/jcs.111.10.1371. PMID 9570755.
- ↑ Graf, Ralph; Christine Daunderer; Manfred Schliwa (April 2000). "Dictyostelium DdCP224 is a microtubule-associated protein and a permanent centrosomal resident involved in centrosome duplication". Journal of Cell Science. 113 ( Pt 10) (10): 1747–58. doi:10.1242/jcs.113.10.1747. PMID 10769206.
- ↑ Whittington, Angela; Oliver Vugrek; Ke Jun Wei; Nortrud G. Hasenbein; Keiko Sugimoto; Madeleine C. Rashbrooke; Geoffrey O. Wasteneys (April 2001). "MOR1 is essential for organizing cortical microtubules in plants". Nature. 411 (6837): 610–613. Bibcode:2001Natur.411..610W. doi:10.1038/35079128. PMID 11385579. S2CID 205017664.
- 1 2 Matthews, Lisa; Philip Carter; Danielle Thierry-Mieg; Ken Kemphues (June 1998). "ZYG-9, A Caenorhabditis elegans Protein Required for Microtubule Organization and Function, Is a Component of Meiotic and Mitotic Spindle Poles". Journal of Cell Biology. 141 (5): 1159–1168. doi:10.1083/jcb.141.5.1159. PMC 2137183. PMID 9606208.
- ↑ Nakaseko, Yukinobu Jeremy; Gohta Goshima; Jun Morishita; Mitsuhiro Yanagida (November 2000). "M phase–specific kinetochore proteins in fission yeast". Current Biology. 11 (8): 537–549. doi:10.1016/S0960-9822(01)00155-5. PMID 11369198. S2CID 18195930.
- ↑ Enke, C.; Zekert, N.; Veith, D.; Schaaf, C.; Konzack, S.; Fischer, R. (March 2007). "Aspergillus nidulans Dis1/XMAP215 Protein AlpA Localizes to Spindle Pole Bodies and Microtubule Plus Ends and Contributes to Growth Directionality". Eukaryotic Cell. 6 (3): 555–562. doi:10.1128/EC.00266-06. PMC 1828926. PMID 17237365.