 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
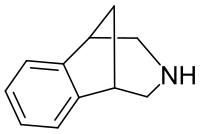
| Formula | C11H13N |
| Molar mass | 159.232 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
2,3,4,5-Tetrahydro-1,5-methano-1H-3-benzazepine is a drug originally researched as a potential opioid analgesic, but was found to be inactive in this assay, and relatively toxic to mice.[1] Subsequently it was found to possess activity as an agonist at nicotinic acetylcholine receptors during the course of work that ultimately led to the discovery of the anti-smoking drug varenicline.[2][3]
More recently this chemical compound is claimed to have been sold as a designer drug under the name A3A, but since the anecdotally reported effects of the product sold under this name do not seem to bear any resemblance to the known pharmacology of genuine 2,3,4,5-tetrahydro-1,5-methano-1H-3-benzazepine, it seems unlikely that this is actually the compound being sold.
See also
References
- ↑ Mazzocchi PH, Stahly BC (April 1979). "Synthesis and pharmacological activity of 2,3,4,5,-tetrahydro-1,5-methano-1H-3-benzazepines". Journal of Medicinal Chemistry. 22 (4): 455–457. doi:10.1021/jm00190a020. PMID 430484.
- ↑ Brooks PR, Caron S, Coe JW, Ng KK, Singer RA, Vazquez E, et al. (2004). "Synthesis of 2,3,4,5-Tetrahydro-1,5-methano-1H-3-benzazepine via Oxidative Cleavage and Reductive Amination Strategies". Synthesis. 2004 (11): 1755–1758. doi:10.1055/s-2004-829135.
- ↑ Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. (May 2005). "Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation". Journal of Medicinal Chemistry. 48 (10): 3474–3477. doi:10.1021/jm050069n. PMID 15887955.