 | |
| Names | |
|---|---|
| IUPAC name
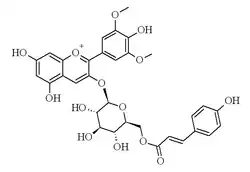
4′,5,7-Trihydroxy-3-{6-O-[(2E)-3-(4-hydroxyphenyl)prop-2-enoyl]-β-D-glucopyranosyloxy}-3′,5′-dimethoxyflavylium | |
| Systematic IUPAC name
(42R,43S,44R,45R,46S,8E)-14,25,27,43,44,45,104-Heptahydroxy-13,15-dimethoxy-7-oxo-21λ4-3,6-dioxa-2(2,3)-[1]benzopyrana-4(2,6)-oxana-1,10(1)-dibenzenadecaphan-8-en-21-ylium | |
| Other names
Malvidin 3-O-(6″-p-coumaroyl)glucoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C32H31O14 | |
| Molar mass | 639.58 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Malvidin-3-O-(6-p-coumaroyl)glucoside is a p-coumaroylated anthocyanin found in grape and wine.[1] There are two forms with the cis and trans isomers of p-coumaric acid. It is a cation.
See also
References
- ↑ Calvo, D.; Sáenz López, R.; Fernández Zurbano, P.; Tena, M. T. (2004). "Migration order of wine anthocyanins in capillary zone electrophoresis". Analytica Chimica Acta. 524 (1–2): 207–213. doi:10.1016/j.aca.2004.06.023.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.