| Pentagonal planar molecular geometry | |
|---|---|
 | |
| Examples | XeF5− |
| Point group | D5h |
| Coordination number | 5 |
| Bond angle(s) | 72° |
| μ (Polarity) | 0 |
In chemistry, the pentagonal planar molecular geometry describes the shape of compounds where five atoms, groups of atoms, or ligands are arranged around a central atom, defining the vertices of a pentagon.
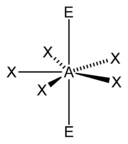
AX5E2
Examples
The only two pentagonal planar species known are the isoelectronic (nine valence electrons) ions XeF−
5 and IF2−
5.[1] Both are derived from the pentagonal bipyramid with two lone pairs occupying the apical positions and the five fluorine atoms all equatorial.
References
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.