 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ECHA InfoCard | 100.029.286 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
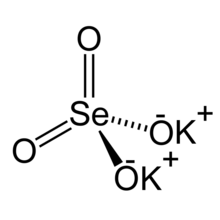
| K 2SeO 4 | |
| Molar mass | 221.2 g/mol[1] |
| Appearance | colorless crystals hygroscopic |
| Odor | odorless |
| Density | 3.07 g/cm3[2] |
| 1.07 g/ml (0 °C) 1.11 g/ml (20 °C) 1.22 g/ml (100 °C) | |
Refractive index (nD) |
1.539 |
| Structure | |
| orthorhombic | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions |
Potassium sulfate |
Other cations |
Sodium selenate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Potassium selenate, K
2SeO
4, is an odorless, white solid that forms as the potassium salt of selenic acid.
Preparation
Potassium selenate is produced by the reaction of selenium trioxide and potassium hydroxide.
- SeO3 + 2 KOH → K2SeO4 + H2O
Alternatively, it can be made by treating selenous acid with potassium hydroxide, followed by oxidation of the resulting potassium selenite with bromine water.[3]
- H2SeO3 + 2 KOH → K2SeO3 + 2 H2O
- K2SeO3 + 2 KOH + Br2 → K2SeO4 + 2 KBr + H2O
Uses
Potassium selenate can be used to produce selenium trioxide.[4] It can also use to treat selenium deficiency in livestock.[5]
References
- ↑ "Potassium Selenate K2SeO4 Molecular Weight". EndMemo. Retrieved 2019-09-03.
- ↑ "Potassium Selenate". American Elements. Retrieved 2019-09-03.
- ↑ Rosenfeld, Irene; Beath, Orville A. (2013). Selenium: Geobotany, Biochemistry, Toxicity, and Nutrition. Elsevier Science. p. 305. ISBN 978-1-4832-7590-1.
- ↑ Sicius, Hermann (2015). Chalkogene : elemente der sechsten hauptgruppe (in German). Springer. p. 28. ISBN 978-3-658-10522-8. OCLC 919684689.
- ↑ Wolfgang Löscher, Angelika Richter, Heidrun Potschka (2014). Pharmakotherapie bei Haus- und Nutztieren (in German). Stuttgart: Enke. ISBN 978-3-8304-1250-2. OCLC 891036290.
{{cite book}}: CS1 maint: multiple names: authors list (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
