 Praseodymium nitrate hydrate | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.711 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Pr(NO3)3 | |
| Molar mass | 326.92 g/mol |
| Appearance | Green crystals |
| Soluble | |
| Solubility | Soluble in amine, ether, and acetonitrile |
| Hazards | |
| GHS labelling: | |
 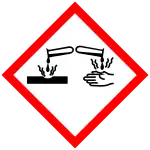  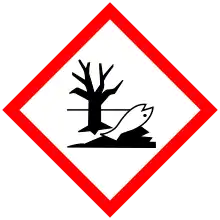 | |
| H272, H302, H315, H318, H410 | |
| P210, P220, P221, P261, P264, P270, P271, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P310, P312, P321, P330, P332+P313, P337+P313, P362, P370+P378, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions |
Praseodymium(III) sulfate |
Other cations |
Neodymium(III) nitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Praseodymium(III) nitrate is a green-colored chemical compound with the chemical formula Pr(NO3)3. It is very hygroscopic and forms a hexahydrate. It is soluble in polar solvents.[1]
Uses
Praseodymium(III) nitrate is used in fluorescent display tubes and phosphors. It is also used in the ultrasonic synthesis of praseodymium molybdate. It also plays a role in the preparation in lanthanide oxysulfides.[1]
References
- 1 2 3 "12909 Praseodymium(III) nitrate hydrate, 99.9% (REO)". Alfa Aesar. Retrieved 3 March 2021.
- ↑ "Praseodymium nitrate". PubChem. Retrieved 3 March 2021.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
