| |||
| Names | |||
|---|---|---|---|
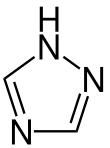
| Preferred IUPAC name
1H-1,2,4-Triazole | |||
| Other names
1,2,4-Triazole pyrrodiazole | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.476 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H3N3 | |||
| Molar mass | 69.00725 | ||
| Appearance | white solid | ||
| Density | 1.439 g/cm3 | ||
| Melting point | 120 to 121 °C (248 to 250 °F; 393 to 394 K) | ||
| Boiling point | 260 °C (500 °F; 533 K) | ||
| very soluble | |||
| Acidity (pKa) | 10,3 | ||
| Basicity (pKb) | 11,8 | ||
| Hazards | |||
| Flash point | 140 °C (284 °F; 413 K) | ||
| Related compounds | |||
Related compounds |
1,2,3-triazole imidazole | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
1,2,4-Triazole (as ligand in coordination compounds, Htrz abbreviation is sometimes used) is one of a pair of isomeric chemical compounds with molecular formula C2H3N3, called triazoles, which have a five-membered ring of two carbon atoms and three nitrogen atoms. 1,2,4-Triazole and its derivatives find use in a wide variety of applications.[1]
Structure and properties
1,2,4-Triazole is a planar molecule. The C-N and N-N distances fall into a narrow range 136 - 132 picometers, consistent with the aromaticity.[2] Although two tautomers can be envisioned, only one exists practically speaking.
1,2,4-Triazole is amphoteric, being susceptible to both N-protonation and deprotonation in aqueous solution. The pKa of 1,2,4-triazolium (C2N3H4+) is 2.45. The pKa of the neutral molecule is 10.26.[3]
Synthesis and occurrence
3_(FIBCEA01).png.webp)
1,2,4-Triazoles can be prepared using the Einhorn–Brunner reaction or the Pellizzari reaction. Unsubstituted 1,2,4-triazole can be prepared from thiosemicarbazide by acylation with formic acid followed by cyclization of 1-formyl-3-thiosemicarbazide into 1,2,4-triazole-3(5)-thiol; oxidation of the thiol by nitric acid or hydrogen peroxide yields 1,2,4-triazole.[5]
1,2,4-Triazoles are featured in many kinds of drugs.[6][7] Notable triazoles include the antifungal drugs fluconazole and itraconazole[8] and the plant growth regulator paclobutrazol.[9] Triazolate is a common bridging ligand in coordination chemistry.[10]
References
- ↑ Potts K. T. (1961). "The Chemistry of 1,2,4-Triazoles". Chemical Reviews. 61 (2): 87–127. doi:10.1021/cr60210a001.
- ↑ Jeffrey, G. A.; Ruble, J. R.; Yates, J. H. (1983). "Neutron diffraction at 15 and 120 K and ab initio molecular-orbital studies of the molecular structure of 1,2,4-triazole". Acta Crystallographica Section B: Structural Science. 39 (3): 388–394. doi:10.1107/S010876818300258X.
- ↑ Garratt, Peter J. (1996). "1,2,4-Triazoles". Comprehensive Heterocyclic Chemistry II. pp. 127–163. doi:10.1016/B978-008096518-5.00080-0. ISBN 9780080965185.
- ↑ Grosjean, Arnaud; Négrier, Philippe; Bordet, Pierre; Etrillard, Céline; Mondieig, Denise; Pechev, Stanislav; Lebraud, Eric; Létard, Jean-François; Guionneau, Philippe (2013). "Crystal Structures and Spin Crossover in the Polymeric Material [Fe(HTRZ)2(TRZ)](BF4) Including Coherent-Domain Size Reduction Effects" (PDF). European Journal of Inorganic Chemistry. 2013 (5–6): 796–802. doi:10.1002/ejic.201201121.
- ↑ C. Ainsworth (1960). "1,2,4-Triazole". Organic Syntheses. 40: 99. doi:10.15227/orgsyn.040.0099.
- ↑ Keri, Rangappa S.; Patil, Siddappa A.; Budagumpi, Srinivasa; Nagaraja, Bhari Mallanna (2015). "Triazole: A Promising Antitubercular Agent". Chemical Biology & Drug Design. 86 (4): 410–423. doi:10.1111/cbdd.12527. PMID 25643871. S2CID 9313180.
- ↑ Kaur, Ramandeep; Ranjan Dwivedi, Ashish; Kumar, Bhupinder; Kumar, Vinod (2016). "Recent Developments on 1,2,4-Triazole Nucleus in Anticancer Compounds: A Review". Anti-Cancer Agents in Medicinal Chemistry. 16 (4): 465–489. doi:10.2174/1871520615666150819121106. PMID 26286663.
- ↑ Kathiravan, Muthu K.; Salake, Amol B.; Chothe, Aparna S.; Dudhe, Prashik B.; Watode, Rahul P.; Mukta, Maheshwar S.; Gadhwe, Sandeep (2012). "The biology and chemistry of antifungal agents: A review". Bioorganic & Medicinal Chemistry. 20 (19): 5678–5698. doi:10.1016/j.bmc.2012.04.045. PMID 22902032.
- ↑ Tesfahun, Wakjira (January 1, 2018). Yildiz, Fatih (ed.). "A review on: Response of crops to paclobutrazol application". Cogent Food & Agriculture. 4 (1): 1–9. doi:10.1080/23311932.2018.1525169. S2CID 134517280.
- ↑ Haasnoot, Jaap G. (2000). "Mononuclear, oligonuclear and polynuclear metal coordination compounds with 1,2,4-triazole derivatives as ligands". Coordination Chemistry Reviews. 200–202: 131–185. doi:10.1016/S0010-8545(00)00266-6.