| |
| Names | |
|---|---|
| Preferred IUPAC name
1,2λ6-Oxathiolane-2,2-dione | |
| Other names
γ-Propane sultone; 1,2-Oxathiolane, 2,2-dioxide; 3-Hydroxyl-1-propane sulfonic acid sulfone; 1-Propane sulfonic acid-3-hydroxyl-γ-sultone; Oxathiolane 2,2-dioxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.013.017 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H6O3S | |
| Molar mass | 122.14 g·mol−1 |
| Appearance | White crystalline solid; colorless liquid above 31 °C |
| Density | 1.392 g/cm3 at 40 °C |
| Melting point | 31 °C (88 °F; 304 K) |
| Boiling point | 112 °C (234 °F; 385 K) at 1.4 mm Hg |
| 10% (20°C)[1] | |
| Hazards | |
| Flash point | 158 °C (316 °F; 431 K) |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
none[1] |
REL (Recommended) |
Ca[1] |
IDLH (Immediate danger) |
Ca [N.D.][1] |
| Safety data sheet (SDS) | NIH.gov |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
1,3-Propane sultone is the organosulfur compound with the formula (CH2)3SO3. It is a cyclic sulfonate ester, a class of compounds called sultones.[2][3] It is a readily melting colorless solid.
Synthesis
It may be prepared by the acid catalyzed reaction of allyl alcohol and sodium bisulfite.
Reactions
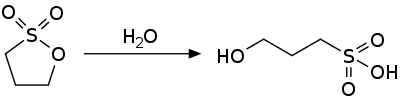
1,3-propane sultone is an activated ester and is susceptible to nucleophilic attack. It hydrolyzes to the hydroxysulfonic acid.
It has been used in the synthesis of specialist surfactants, such as CHAPS detergent.[4]
Safety
Typical of activated esters, 1,3-propane sultone is an alkylating agent. 1,3-Propane sultone is toxic, carcinogenic, mutagenic, and teratogenic.[5][6]
See also
References
- 1 2 3 4 NIOSH Pocket Guide to Chemical Hazards. "#0525". National Institute for Occupational Safety and Health (NIOSH).
- ↑ R. J. Cremlyn (1996). An Introduction to Organosulfur Chemistry. Chichester: John Wiley and Sons. ISBN 0-471-95512-4.
- ↑ Morimoto, Yoshiki; Kurihara, Hajime; Kinoshita, Takamasa (2000). "Can α-sultone exist as a chemical species? First experimental implication for intermediacy of α-sultone" (PDF). Chemical Communications (3): 189–190. doi:10.1039/A909094K.
- ↑ Hjelmeland, LM (November 1980). "A nondenaturing zwitterionic detergent for membrane biochemistry: design and synthesis". Proceedings of the National Academy of Sciences of the United States of America. 77 (11): 6368–70. Bibcode:1980PNAS...77.6368H. doi:10.1073/pnas.77.11.6368. PMC 350285. PMID 6935651.
- ↑ "Scorecard Chemical Profile for Propane Sultone". Archived from the original on 2008-09-23. Retrieved 2008-11-17.
- ↑ "NIOSH Pocket Guide to Chemical Hazards". Retrieved 2013-11-13.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.