 | |
 | |
| Names | |
|---|---|
| IUPAC name
11β,18,21-Trihydroxypregn-4-ene-3,20-dione | |
| Systematic IUPAC name
(1S,3aS,3bS,9aR,9bS,10S,11aR)-10-Hydroxy-1-(hydroxyacetyl)-11a-(hydroxymethyl)-9a-methyl-1,2,3,3a,3b,4,5,8,9,9a,9b,10,11,11a-tetradecahydro-7H-cyclopenta[a]phenanthren-7-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.384 |
| MeSH | 18-hydroxycorticosterone |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H30O5 | |
| Molar mass | 362.46 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
18-Hydroxycorticosterone is an endogenous steroid.[1][2] It is a derivative of corticosterone.[3][4][5]
Function
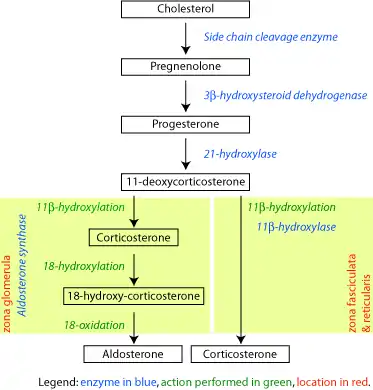
Corticosteroid biosynthetic pathway in rat
18-Hydroxycorticosterone serves as an intermediate in the synthesis of aldosterone by the enzyme aldosterone synthase in the zona glomerulosa.
See also
References
- ↑ Reddish MJ, Guengerich FP (August 2019). "Human cytochrome P450 11B2 produces aldosterone by a processive mechanism due to the lactol form of the intermediate 18-hydroxycorticosterone". The Journal of Biological Chemistry. 294 (35): 12975–12991. doi:10.1074/jbc.RA119.009830. PMC 6721951. PMID 31296661.
- ↑ Mulatero P, di Cella SM, Monticone S, Schiavone D, Manzo M, Mengozzi G, Rabbia F, Terzolo M, Gomez-Sanchez EP, Gomez-Sanchez CE, Veglio F (March 2012). "18-hydroxycorticosterone, 18-hydroxycortisol, and 18-oxocortisol in the diagnosis of primary aldosteronism and its subtypes". The Journal of Clinical Endocrinology and Metabolism. 97 (3): 881–9. doi:10.1210/jc.2011-2384. PMID 22238407.
- ↑ Gupta V (October 2011). "Mineralocorticoid hypertension". Indian Journal of Endocrinology and Metabolism. 15 Suppl 4 (8): S298–312. doi:10.4103/2230-8210.86972. PMC 3230101. PMID 22145132.
- ↑ Freel EM, Shakerdi LA, Friel EC, Wallace AM, Davies E, Fraser R, Connell JM (September 2004). "Studies on the origin of circulating 18-hydroxycortisol and 18-oxocortisol in normal human subjects". The Journal of Clinical Endocrinology and Metabolism. 89 (9): 4628–33. doi:10.1210/jc.2004-0379. PMC 1283128. PMID 15356073.
- ↑ Izumi Y (July 2010). "[18-Hydroxycorticosterone (18-OH-B)]". Nihon Rinsho. Japanese Journal of Clinical Medicine (in Japanese). 68 (Suppl 7): 348–53. PMID 20960793.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.