 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Ethyl-4,5-dihydro-1,3-oxazole | |
| Other names
2-Ethyloxazoline | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.817 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H9NO | |
| Molar mass | 99.133 g·mol−1 |
| Density | 0.982 g/mL[1] |
| Melting point | −62 °C (−80 °F; 211 K)[1] |
| Boiling point | 128.4 °C (263.1 °F; 401.5 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
2-Ethyl-2-oxazoline (EtOx) is an oxazoline which is used particularly as a monomer for the cationic ring-opening polymerization to poly(2-alkyloxazoline)s.[2] This type of polymers are under investigation as readily water-soluble and biocompatible materials for biomedical applications.[3]
Production
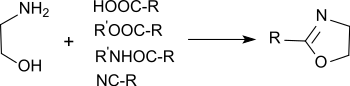
From propionic acid and derivatives
Carboxylic acids, carboxylic esters, carboxylic amides and nitriles can react with 2-amino alcohols at 200 °C upon dehydration to the corresponding N-(2-hydroxy)carbamide, which react further at 260–280 °C upon dehydration to the 2-alkyl-2-oxazoline.
 Synthese von 2-Ethyl-2-oxazolin aus Carbonsäurederivaten
Synthese von 2-Ethyl-2-oxazolin aus Carbonsäurederivaten
For example N-(2-hydroxyethyl)propionamide is first formed from propionic acid and ethanolamine in 74% yield which can be dehydrated to give 2-ethyl-2-oxazoline in about 75% yield.[4]
propionamid.svg.png.webp) Synthese von 2-Ethyl-2-oxazolin über N-(2-Hydroxyethyl)propionamid
Synthese von 2-Ethyl-2-oxazolin über N-(2-Hydroxyethyl)propionamid
Less drastic reaction conditions require the dehydration of the N-(2-hydroxyethyl)propionamide in vacuo in the presence of iron(III)chloride, which delivers the product in 90% yield.[5] An even higher yield of 96.2% is obtained by heating with zinc acetate.[6]
An economic one-pot reaction is heating the salt of propionic acid with ethanolamine at 200 °C in vacuo in the presence of zinc chloride yielding 82% 2-ethyl-2-oxazoline.[6] From the water-containing distillate pure 2-ethyl-2-oxazoline can be isolated by extraction with diethylbenzene and subsequent distillation[6] or by distillation only after addition of diethyl phosphite or dimethyldichlorosilane. The product can be dried to a residual water content of 10 ppm.[7]
In another one-pot reaction propionic acid is converted first with 2-aminoethanol to 2-hydroxyethylamide, than reacted with boric acid at 130 °C yielding a boric acid ester which is finally thermolyzed at 280 °C in 92% yield to 2-ethyl-2-oxazoline.[8]
 Synthese von 2-Ethyl-2-oxazolin über Boratester
Synthese von 2-Ethyl-2-oxazolin über Boratester
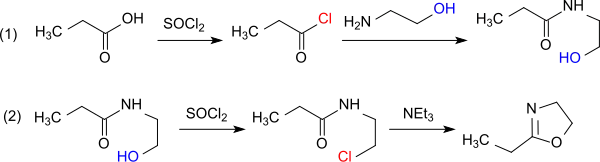
From propionic acid and thionyl chloride can be obtained propanoyl chloride, which reacts with ethanolamine in the presence of an acid scavenger (for example pyridine) to N-propionyl-2-aminoethanol. With further thionyl chloride this reacts further to 2-chloroethylamide. With the chloride ion as a better leaving group, this intermediate is cyclized by simple heating to the oxazoline. Water must be excluded due to the tendency of oxazolines towards ring-opening by chloride ions during protonation of the imine nitrogen.[9]
 Synthese von 2-Ethyl-2-oxazolin über Propionsäureäurechlorid
Synthese von 2-Ethyl-2-oxazolin über Propionsäureäurechlorid
The direct reaction of propanoyl chloride with 2-chloroethylamine hydrochloride in the presence of triethylamine avoids the formation of water.
 Synthese von 2-Ethyl-2-oxazolin mit Chlorethylamin
Synthese von 2-Ethyl-2-oxazolin mit Chlorethylamin
From propanal
Propanal reacts with 2-aminoethanol in t-butanol to 2-ethyl-2-oxazoline in the presence of the iodinating reagent 1,3-diiodo-5,5-dimethylhydantoin (DIH) and potassium carbonate.[10]
 Synthese von 2-Ethyl-2-oxazolin aus Propionaldehyd
Synthese von 2-Ethyl-2-oxazolin aus Propionaldehyd
Properties
2-Ethyl-2-oxazoline is a readily water-soluble, colorless liquid which is also soluble in a variety of organic solvents and possesses an amine-like smell.[11] Aqueous solutions react alkaline. The compound is stable in alkaline but hydrolyses under acid action.
Applications
In anhydrous form, 2-ethyl-2-oxazoline is mostly used as a monomer.[2]
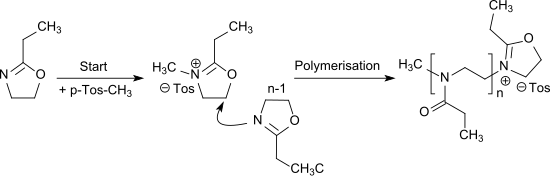
The cationic ring-opening polymerization of 2-ethyl-2-oxazoline[12] can be initiated by alkylation with e.g. methyl tosylate or triflates (in particular methyl triflate) and leads to the water-soluble poly(2-ethyl-2-oxazoline) which is a propionyl-substituted linear polyethylenimine and can also be seen as a pseudo-polypeptide.[13]
 Kationische lebende Polymerisation von 2-Ethyl-2-oxazolin
Kationische lebende Polymerisation von 2-Ethyl-2-oxazolin
The polymerization of 2-ethyl-2-oxazoline can also be carried out as living cationic polymerization.[14]
Copolymers with other 2-alkyl-2-oxazolines[15] and other monomers[16] allow the preparation of random copolymers and block copolymers.
The copolymers obtained can be used as biocompatible drug carriers,[17] in coatings and adhesives, and in many other applications.[18]
The elimination of the propionyl group from poly (2-ethyl-2-oxazoline) yields linear polyethyleneimine.[19][20]
 Herstellung von Linearem Polyethylenimin aus Poly(2-ethyloxazolin)
Herstellung von Linearem Polyethylenimin aus Poly(2-ethyloxazolin)
References
- 1 2 3 "2-Ethyl-2-oxazoline". Sigma-Aldrich.
- 1 2 T. Kagiya; S. Narisawa; T. Maeda; K. Fukui (1966), "Ring-opening polymerization of 2-substituted 2-oxazolines", J. Polym. Chem., Polym. Lett., vol. 4, no. 7, pp. 441–445, doi:10.1002/pol.1966.110040701
- ↑ R. Hoogenboom (2009), "Poly(2-oxazoline)s: A polymer class with numerous potential applications", Angew. Chem. Int. Ed., vol. 48, no. 43, pp. 7978–7994, doi:10.1002/anie.200901607, PMID 19768817
- ↑ H. Wenker (1935), "The synthesis of Δ2-oxazolines and Δ2-thiazolines from N-acyl-2-aminoethanols", J. Am. Chem. Soc., vol. 57, no. 6, pp. 1079–1080, doi:10.1021/ja01309a034
- ↑ US 4203900, M.E. Kaiser, "Process for preparing 2-oxazolines", published 1980-05-20, assigned to The Dow Chemical Co.
- 1 2 3 US 4354029, M.E. Kaiser, D.L. Larson, "Preparation of 2-substituted 2-oxazolines with organic zinc salt catalysts", published 1982-10-12, assigned to The Dow Chemical Co.
- ↑ US 4281137, J.W. Sanner, P.W. Owen, "Purification of 2-oxazolines", published 1981-07-28, assigned to The Dow Chemical Co.
- ↑ B. Ilkgul; D. Gunes; O. Sirkecioglu; N. Bicak (2010), "Synthesis of 2-oxazolines via boron esters of N-(2-hydroxyethyl) amides", Tetrahedron Lett., vol. 51, no. 40, pp. 5313–5315, doi:10.1016/j.tetlet.2010.07.167
- ↑ M.N. Holerca; V. Percec (2000), "1H NMR Spectroscopic Investigation of the Mechanism of 2-Substituted-2-Oxazoline Ring Formation and of the Hydrolysis of the Corresponding Oxazolinium Salts", Eur. J. Org. Chem., vol. 2000, no. 12, pp. 2257–2263, doi:10.1002/1099-0690(200006)2000:12<2257::AID-EJOC2257>3.0.CO;2-2
- ↑ S. Takahashi; H. Togo (2009), "An Efficient Oxidative Conversion of Aldehydes into 2-Substituted 2-Oxazolines Using 1,3-Diiodo-5,5-dimethylhydantoin", Synthesis, vol. 2009, no. 14, pp. 2329–2332, doi:10.1055/s-0029-1216843
- ↑ 2-Ethyl-2-oxazoline at AlfaAesar, accessed on 5. Juni 2016 (PDF) (JavaScript required).
- ↑ B.L. Rivas; S.I. Ananias (1987), "Ring-opening polymerization of 2-ethyl-2-oxazoline", Polym. Bull., vol. 18, no. 3, pp. 189–194, doi:10.1007/BF00255109, S2CID 94946485
- ↑ H. Schlaad; R. Hoogenboom (2012), "Poly(2-oxazoline)s and Related Pseudo-Polypeptides", Macromol. Chem. Rapid Commun., vol. 33, no. 19, p. 1599, doi:10.1002/marc.201200571, PMID 22965791
- ↑ C. Guerrero-Sanchez; R. Hoogenboom; U.S. Schubert (2006), "Fast and "green" living cationic ring opening polymerization of 2-ethyl-2-oxazoline in ionic liquids under microwave irradiation", Chem. Commun., vol. 36, no. 36, pp. 3797–3799, doi:10.1039/B608364A, PMID 16969461
- ↑ M. Glassner; K. Lava; V.R. de la Rosa; R. Hoogenboom (2014), "Tuning the LCST of poly(2-cyclopropyl-2-oxazoline) via gradient copolymerization with 2-ethyl-2-oxazoline", Polym. Chem., vol. 52, no. 21, pp. 3118–3122, doi:10.1002/pola.27364
- ↑ S. Motokucho; M. Furukawa; M. Kawashima; K. Kojio; K. Yoshinaga (2013), "Physical properties of poly(tetrahydrofuran)-block-poly(2-ethyl-2-oxazoline) triblock copolymer", Polym. J., vol. 45, no. 11, pp. 1115–1119, doi:10.1038/pj.2013.39
- ↑ V.R. de la Rosa (2014), "Poly(2-oxazoline)s as materials for biomedical applications", J. Mater. Sci. Mater. Med., vol. 25, no. 5, pp. 1–15, doi:10.1007/s10856-013-5034-y, PMID 23975334, S2CID 1766066
- ↑ "Etox, 2-Ethyl-2-Oxazoline, Product Information Sheet". Polymer Chemistry Innovations, Inc. Retrieved 2016-07-19.
- ↑ US 20100197888, A. Adib, F. Stock, P. Erbacher, "Method for manufacturing linear polyethyleneimine (PEI) for transfection purpose and linear PEI obtained with such method", published 2010-08-05, assigned to Polyplus Transfection
- ↑ H.M.L. Lambermont-Thijs; F.S. van der Woerdt; A. Baumgaertel; L. Bonami; F.E. Du Prez; U.S. Schubert; R. Hoogenboom (2010), "Linear Poly(ethylene imine)s by Acidic Hydrolysis of Poly(2-oxazoline)s: Kinetic Screening, Thermal Properties, and Temperature-Induced Solubility Transitions", Macromolecules, vol. 43, no. 2, pp. 927–933, doi:10.1021/ma9020455